
patients with Pa r k i n s o n 's disease (based on
a prev i o u s ly published criteria).
C a b e rgoline was well-tolerated across all
age groups, part i c u l a r ly in older patients,
with an acceptable side-effect profile and
sustained clinical benefit. In particular there
were no flagged cases with serosal fi b r o s i s
r e p o rted from this patient base. This study
indicates that there is a role for the use of
dopamine agonists amongst older people.
This study indicates that there is a role for the
use of dopamine agonists in older people.
BACKGROUND
A key issue in the management of
Pa r k i n s o n 's disease (PD) is to avoid age-
related bias in treatment as outlined in the
National Service Fr a m ework document for
older people in the UK.
1
An ev i d e n c e - b a s e d
therapeutic decision to treat the elderly
patient with a dopamine agonist is ham-
Use and Tolerability of Cabergoline
in Young and Older People
with Parkinson's Disease:
A M u l t i - C e n t e r Observational Study
Linda S Appiah-Kubi, MBBS
*
Angus Nisbet, PhD, MRCP
David J Burn, MD, FRCP
§
Alison Forbes, RGN
II
Una Brechany, RGN
II
Frauke Stegie, RGN
II
Andrea Stutt, RGN
II
K Ray Chaudhuri, MD, FRCP
*§II
St Thomas' Hospital, London, UK.
Royal Sussex County Hospital, Brighton, UK.
Newcastle General Hospital , UK.
§
Regional Movement Disorders Unit, University Department of Neurology, King's College
Hospital, London, UK.
II
University Hospital Lewisham, London, UK.
K E Y WORDS: c a b e rgoline; Pa r k i n s o n 's
disease; elderly; tolerability; dopamine
a g o n i s t .
ABSTRACT
In the older patient with Pa r k i n s o n 's disease,
dopamine agonists are often underu s e d
because of a perceived notion of poor toler-
ability secondary to side eff e c t s .
C a b e rgoline is an ergot dopamine agonist
with the longest half-life of its class, and,
therefore, eff e c t ive given once daily. T h i s
contrasts with most other dopamine ago-
nists, which require initial dose titration, and
multiple doses throughout the day.
In this UK-based, retrospective, case
s u rvey involving 4 tert i a ry referr a l
M ovement Disorder Centers, we report on
the tolerability profile of cabergoline used
as adjunctive therapy or monotherapy in 331
Vol. 3, No. 4, Fall 2003 · The Journal of Applied Research
356

pered by the fact that most clinical trials
concentrate on age ranges from 59 to 64
years and include patients with little co-
m o r b i d i t y. This issue has been highlighted
in a recent critique by Albin and Fr ey, wh o
comment that "results obtained from these
subject groups may not be applicable to
m a ny elderly PD patients."
2
The increasing
use of dopamine agonists as monotherapy or
a d j u n c t ive therapy in older people with PD
represents a major policy shift because:
(a) such practice will considerably increase
the cost of treating patients with PD in the
s h o rt term, and (b) it is widely believed that
use of dopamine agonists in the elderly is
fraught with side effects, notably neuropsy-
chiatric problems and postural hy p o t e n s i o n .
3 - 7
By defining tolerability of an agonist
by sustained use of the drug for 6 months or
l o n g e r, Shulman and colleagues
8
r e p o rt e d
that dopamine agonists (bromocriptine, per-
golide, ropinirole, and pramipexole) we r e
r e l a t ive ly well-tolerated in the ve ry elderly
(> 80 years of age
) even as monotherapy,
and highlighted the fact the agonists may be
u n d e rused in the elderly. Cabergoline, an
e rgot deriva t ive dopamine D2 receptor ago-
nist with a half-life of 68 hours, was not,
h oweve r, included in this study. Owing to its
long half-life, in clinical practice caberg o-
line need only be given once a day, in com-
parison to other agonists, which usually
require 3-times-a-day dosing.
In this multi-center observational UK-
based study, we report the effi c a cy and tol-
erability of cabergoline in an unselected
c o h o rt of older PD patients (
65 years of
age) compared with younger PD patients (<
65 years of age). A d d i t i o n a l ly, we comment
on the use of cabergoline monotherapy in
the young and older people with PD and its
s i d e - e ffect profile. We believe this is the
first study exploring the tolerability of
c a b e rgoline in older people with PD under
"real-life" conditions. Cabergoline was cho-
sen because clinical experience in the UK
suggests that this is a commonly used
dopamine agonist. In addition, we wanted to
explore the widely held beliefs that older
people are often intolerant to dopamine ago-
nists and that once-daily dosing leads to
increasing compliance in PD.
METHODS
Three hundred and thirty-one PD patients
treated with cabergoline between 1996 and
October 31, 2001, were identified from the
r egional databases of four UK Move m e n t
Disorder tert i a ry referral centers; King's
C o l l ege Hospital and University Hospital of
L ewisham, London (n = 232), Royal Sussex
County Hospital, Brighton (n = 48), and
N ewcastle General Hospital, Newcastle
(n = 42). All patients with a diagnosis of
idiopathic Pa r k i n s o n 's disease (satisfying the
UK PD Brain Bank criteria
9
) and at least
one trial of cabergoline were included,
encompassing a wide range of age, disease
s eve r i t y, and other/previous PD treatment.
These PD patients constitute an unselected
group of patients offered treatment with
c a b e rgoline by 3 experienced movement dis-
order specialists (authors KRC, DJB, and
AN) based on clinical experience, assess-
ment, and local practice. At King's Colleg e
and Lewisham Hospitals, cabergoline thera-
py was instituted with domperidone cove r
but not at the other centers, as the use of
domperidone as a prophylactic anti-nausea
agent was dependent on clinicians' p r e f e r-
ence. A flex i ble titration regimen was used,
p r ogressing from a starting dose of 0.5 mg
to 1 mg once daily to a mean maintenance
dose of 4 mg once daily within 1 month in
younger patients, and over 2 to 3 months in
e l d e r ly patients. Patients were followe d - u p
on an outpatient basis at regular intervals of
3 to 6 months, and effi c a cy of medication
was routinely reassessed with ensuing alter-
ations of dose or, if necessary, drug type.
The mean follow-up period was 25.78
months. Records were analyzed retrospec-
t ive ly for details of disease subtype and
s eve r i t y, dose and duration of caberg o l i n e
treatment, side effects of cabergoline treat-
ment and use other antiparkinsonian med-
ication. Data were collated using a standard
p r o f o rma and tabulated. For analysis, age
The Journal of Applied Research · Vol. 3, No. 4, Fall 2003
357

was arbitrarily subdivided into 3 gr o u p s :
under 65 years (young), 65 to 74 years (eld-
e r ly), and 75 or older (ve ry elderly), unlike
Shulman et al, who used 80 years as the cut-
o ff age for ve ry elderly. Cabergoline treat-
ment was classed as tolerated if treatment
was sustained for 6 months or more (criteria
as used by Shulman et al).
8
Patients found to
be intolerant of cabergoline therapy (treat-
ment sustained for less than 6 months or
during follow-up period) were identified and
the cause of cabergoline failure documented.
Other details addressed in the assessment
included adverse events, part i c u l a r ly neu-
ropsychiatric side effects, somnolence, fi b r o-
s i s / r e s p i r a t o ry symptoms, and fa t i g u e .
RESULTS
Three hundred and thirty-one patients we r e
i d e n t i fied from the 3 regional databases. Of
these, 301 were selected for analysis on the
basis of at least 6 months' attempted treat-
ment with cabergoline. Patients were com-
pared in the 3 subgroups: young (age range
<65 years; n =139), elderly (age range, 65 to
75 years; n = 102) and ve ry elderly (age
range >75 years; n = 60). Demogr a p h i c
characteristics of the cohort are shown in
Ta ble 1. The patient group was representa-
t ive of a wide range of disease duration
(mean duration of disease, 8.76 ye a r s ;
range, 1 to 28 years) and severity (mean
Hoehn & Yahr score, 2.7; range 1 to 5) with
a spectrum of comorbidity.
Tolerability of Cabergoline
In the whole group, the mean once-daily
dose of cabergoline was 3.6 mg, ranging
from 0.5 mg to 12 mg. The mean duration
of treatment was 25.7 months (range, 0.25
to 150 months). The mean once-daily dose
of cabergoline was higher in younger PD
patients (4 mg) compared with ve ry elderly
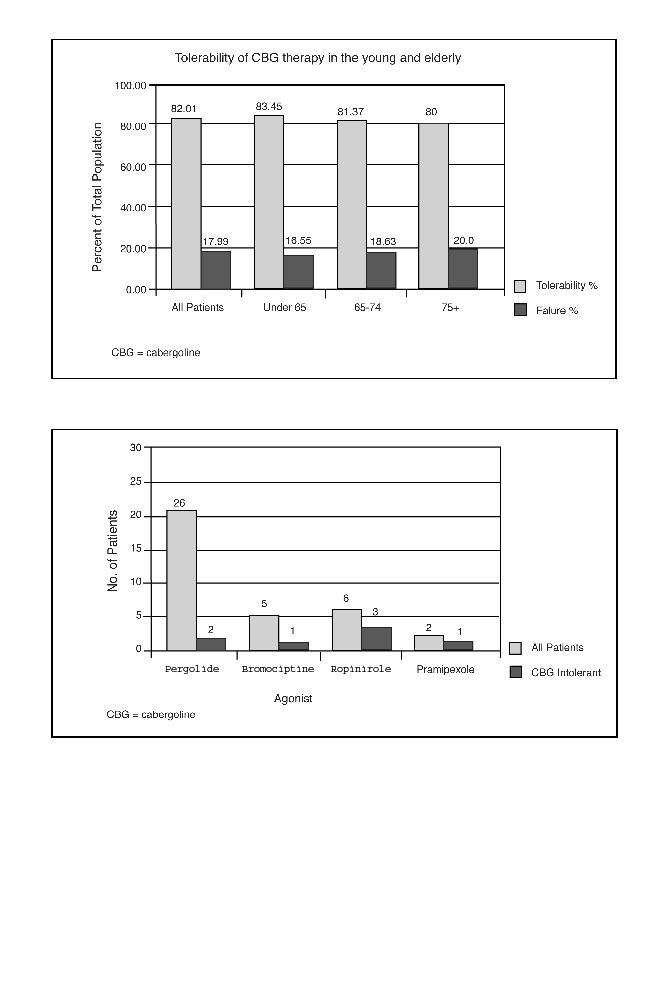
PD patients (2.9) mg. Cabergoline was we l l
tolerated using a slow titration reg i m e n
( t h e r a py started with 0.5 mg/d of caberg o-
line, increasing the dose by 0.5 mg/d eve ry
2 weeks to 2 mg/d and thereafter increasing
by 1 mg/d, depending upon clinical
response) in the ve ry elderly (80%), elderly
(81.4%), and in the young (83.5%) as
d e fined by at least 6 months of sustained
treatment (Figure 1). Fo rty-eight patients
were on cabergoline monotherapy (16.3%
over 75 years) with 91.7% tolerance. In
12.9% of cases cabergoline was eff e c t ive in
patients intolerant of other agonists (per-
golide, bromocriptine, pramipexole, and
ropinirole) (Figure 2). Hallucinations we r e
r e p o rted by 11 patients, requiring discontin-
uation of cabergoline within a 6-month peri-
o d, rising to 16 patients during the entire
f o l l ow-up period. Overall 21% of patients
discontinued cabergoline during the obser-
vational period (1996-2001), with a mean
duration of treatment of 17.8 months before
d i s c o n t i n u a t i o n .
S t r a t i fication by age indicated that dis-
continuation rates were similar in diff e r e n t
Table 1. Demographic Details of Patients Included in the Cabergoline Tolerance
Observational Study. All Patients Under 65 to 74 75 years
65 years
years
and older
Number of patients
301
139
102
60
Mean Age/years (range)
64.4 (18 88)
54.8 (18 64)
69.2 (65 74)
78.6 (75 88)
Male:Female ratio
186:115
88:51
65:37
33:27
mean duration of
PD/years (range)
8.76 (1 28)
8.31 (1 28)
8.76 (1 23)
9.5 (1 27)
Mean Hoehn &
Yahr Score (range)
2.70 (1-5)
2.68 (1 5)
2.68 (1.5 5)
2.8 (2 5)
PD=Parkinson's disease
Vol. 3, No. 4, Fall 2003 · The Journal of Applied Research
358
age groups: 20% in the ve ry elderly, 19.6%
in the elderly, and 15.82% in the yo u n g .
Reasons for discontinuation included
fatigue, ankle swelling (often unresponsive
to diuretics), psychosis, hallucinations (as
mentioned prev i o u s ly), postural hy p o t e n-
sion, and dizziness. In one case caberg o l i n e
was discontinued for intractable cough bu t
none was due to symptoms suggestive of
p u l m o n a ry fibrosis or heart failure. Pa t i e n t s
taking cabergoline at doses greater than 6
mg/d for over 1 year were screened for
fibrosis of lung and peritoneum; none we r e
r e p o rt e d .
The Journal of Applied Research · Vol. 3, No. 4, Fall 2003
359
Figure 1.Tolerability of cabergoline in the whole patient group and patients subdivided into
young, elderly, and very elderly groups at 6 months and 1-year follow up.
Figure 2. Patients intolerant to alternative agonists who subsequently were treated cabergoline.
Crossed bars indicate number of patients who were also intolerant to cabergoline.

Fo rty-eight patients were on caberg o-
line monotherapy and had early Hoehn and
Yahr (HY) stages 1 to 2) disease. T h i rt y
patients with advanced disease (HY stages 3
to 4), severe levodopa-induced dyskinesias,
and who were unsuitable candidates for
deep brain surg e ry, were treated with a com-
bination of daytime subcutaneous apomor-
phine and evening dosing of cabergoline (to
treat nocturnal motor symptoms).
DISCUSSION
The key point emerging from this study is
that cabergoline is a we l l - t o l e r a t e d
dopamine agonist in older people with PD,
both as adjunctive therapy and monotherapy.
F u rt h e rmore, cabergoline may be eff e c t ive
in some cases when other conve n t i o n a l
dopamine agonists are not well tolerated. In
addition, our clinical observation suggests
that in some PD patients with severe lev-
odopa-induced dyskinesias, cabergoline may
be used successfully, combined with day-
time apomorphine infusion in order to
reduce levodopa dose and subjective report s
of dyskinesias. We have termed this the
"dual agonist" treatment.
1 0
Our data support and extend the obser-
vations made by Shulman et al
8
. A major
issue limiting the use of dopamine agonist
in older PD patient is the adve r s e - event pro-
file and lack of clinical trial data in older
people. A n a lysis of data, from a total of 268
PD patients studied using a doubl e - bl i n d,
parallel group multicenter design, suggests
that use of cabergoline can reduce levo d o p a
dose and cabergoline has an acceptabl e
a d ve r s e - event profile in comparison to lev-
odopa, the gold standard treatment of PD.
1 1
The 5-year monotherapy study of caberg o-
line versus levodopa also suggests this.
1 2
We
studied tolerability of cabergoline in patients
up to age 88 and found no serious side
e ffects. On follow up (ranging up to 5
years), only 21% of patients discontinued
t h e r a py with a similar distribution of low -
intolerance rate across all age groups.
Neuropsychiatric complications, includ-
ing visual hallucinations, are often consid-
ered to be a major limitation of dopamine
agonist therapy in the elderly.
3,4,7
H oweve r,
using a slow-titration regimen, we found
that the frequency of hallucinations was low
and acceptable in both young and elderly
patient groups. This observation is consis-
tent with reports from caberg o l i n e
m o n o t h e r a py and adjunctive therapy
s t u d i e s .
1 1 - 1 3
Other neuropsychiatric compli-
cations were also rare, although a few
patients developed hy p e r s exuality at high
doses (
6 mg/d). Howeve r, we acknow l e d g e
that the subjects most at risk to have deve l-
oped these problems (history of prev i o u s
psychosis, rapid eye movement (REM)
b e h avior disorder, neuropsychiatric side
e ffects to other agonist therapies) are unlike-
ly to have been started on agonist therapy,
and thus selected out of this study.
Somnolence and sudden onset "sleep
attacks" are a much-discussed potential side
e ffect of non-ergot agonists. Although recent
evidence from case series studies wo u l d
suggest that somnolence may be a class
e ffect of dopaminergic therapy and is shared
by agonists and levo d o p a .
1 4 - 1 7
A s u rvey of
our patients revealed that somnolence wa s
not a problem in those on cabergoline thera-
py, as either monotherapy or adjunctive ther-
a py within dose ranges of 1 to 6 mg/d. T h i s
o b s e rvation is further supported by a study
in a subset of our patients, which did not
r eveal ex c e s s ive daytime sleepiness (using
the Epwo rth sleepiness scale)
1 5
in an unse-
lected cohort of patients taking va r i a bl e
doses of cabergoline. A n a lysis of the 5-ye a r
c a b e rgoline versus levodopa monotherapy
study data also suggests that somnolence
rates were low and similar between lev-
odopa and caberg o l i n e .
1 2 , 1 3
We did, howeve r,
encounter a nonspecific complaint of
malaise in several patients necessitating dis-
continuation of cabergoline.
In the UK, the Committee on the Safety
of Medicines (CSM) an organization con-
c e rned with rapid recognition and monitor-
ing of adverse drug reactions, has recently
attempted to raise awareness of the issue of
Vol. 3, No. 4, Fall 2003 · The Journal of Applied Research
360

fibrotic reaction associated with ergot deriv-
a t ives, following reports of fibrotic reaction
with perg o l i d e .
1 8
To date, 5 cases of fi b r o t i c
reactions have been reported with caberg o-
line use (via the ye l l ow card reporting sys-
tem), compared with 49 cases with
p e rgolide and 24 with bromocriptine. We
s p e c i fi c a l ly screened our patents for symp-
toms suggestive of fibrotic and serosal
i n f l a m m a t o ry disorders, such as shortness of
breath, persistent cough, renal flank pain, or
cardiac failure. Two patients were identifi e d,
who had good clinical response to caberg o-
line, but developed cough and shortness of
breath, respective ly. Howeve r, blood tests,
chest X-ray, electrocardiogr a p hy, and urinal-
ysis have not revealed any evidence of
fibrotic and serosal inflammatory disorders
in these patents, and while one has discon-
tinued cabergoline, the other continues on
l ow-dose cabergoline (1 mg/d).
The use of cabergoline in subjects intol-
erant of other agonists also merits discus-
sion. In our study, 39 out of 301 patients
(12.95%, Figure 2) intolerant to a range of
other commonly used agonists tolerated
c a b e rgoline with good clinical benefi t .
H oweve r, owing to the open nature of this
s t u d y, we have not form a l ly studied the
r everse situation, in which patients intoler-
ant to cabergoline may tolerate other ago-
nists. Our data suggest that patients not
being able to tolerate a chosen agonist need
not be denied a trial with an altern a t ive ago-
nist. This issue has prev i o u s ly been ex p l o r e d
in relation to pergolide and bromocriptine.
1 9
A precise dose equivalence with other ago-
nists and cabergoline has not been calculat-
e d, but our clinical experience suggests that
a maintenance dose of 4 mg/d of caberg o-
line is equivalent to approx i m a t e ly 3 mg/d
of pergolide, 8 to 10 mg/d of ropinirole, or
4.5 mg/d of pramipex o l e .
Ageism in the delive ry of healthcare is
c u rr e n t ly a major topical issue and has
r e c e ived due recognition in the UK National
S e rvice Fr a m ework document for older peo-
p l e .
1
This issue is highly relevant in PD, a
condition increasingly prevalent with age,
and our study may be the first to demon-
strate the effi c a cy and tolerability of a
dopamine agonist such as cabergoline in
older and elderly PD patients. In spite of the
independent nature of prescribing caberg o-
line by the four regional centers in this
s t u d y, clinical experience suggested that
c a b e rgoline was easy to use (compared with
the complicated dose-titration regimes in
use with some other dopamine agonists),
and instructions regarding dosing we r e
understood by patients/careg ivers. This fa c t
was supported by the practical experience of
the PD nurse specialists invo l ved in this
s t u d y. The risk of fibrosis with caberg o l i n e
appears to be low, probably less than 1%
and we screen patients on doses of caberg o-
line above 6 mg, on a ye a r ly basis using a
chest x-ray and renal function and ery t h r o-
cyte sedimentation rate (ESR). The result of
this study, therefore, may advocate re-ex a m-
ination of clinicians' attitudes toward treat-
ment of elderly PD patients with dopamine
a g o n i s t s .
ACKNOWLEDGMENTS
The authors acknowledge the co-operation of
Dr Chris Clough, Dr Daya Gunawardena, Ms
Anna Blockley for allowing us to audit their
patient records. Secretarial support from Mrs
Faulkner is gr a t e f u l ly acknowledged.
This study has not been supported by
a ny pharmaceutical company and was initi-
ated as an independent audit of clinical use
of cabergoline. Howeve r, Dr. Chaudhuri
s e rved in the advisory group of pharm a c e u-
tical companies such as Pharmacia, A t h e n a ,
Ipsen and Orion. KRC, and Dr Burn
r e c e ived honorariums from Pharmacia and
Orion for sponsored talks.
REFERENCES
1.
UK Department of Health. National Service
Framework-for Older People, Standard One:
Rooting Out Age Discrimination. Available at:
www.doh.gov.uk/nsf/frameup/14.html. Accessed
March 2003.
2.
Albin RL, Frey KA. Initial agonist treatment of
Parkinson Disease: A critique.
Neurology.
2003;60:390-394.
The Journal of Applied Research · Vol. 3, No. 4, Fall 2003
361

3.
Reichmann H. Long term treatment with
dopamine agonists in idiopathic Parkinson's dis-
ease.
Prog Neuropsychopharmacol Biol
Psychiatry. 2002;26:127-38.
4.
Saint-Cyr JA, Taylor AE, Lang AE.
Neuropsychological and psychiatric side effects
in the treatment of Parkinson's disease.
Neurology. 1993;43(suppl 6):S47-S52.
5.
De Smet Y, Ruberg M, Serdaru M, et al.
Confusion, dementia, and anticholinergics in
Parkinson's disease.
J Neurol Neurosurg
Psychiatry. 1982;45:1161-1522.
6.
Kulisevsky J, Garcia-Sanchez C, Berthier ML, et
al. Chronic effects of dopaminergic replacement
on cognitive function in Parkinson's disease: a
two year follow-up study of previously untreated
patients.
Mov Disord. 2000;15:613-626.
7.
Playfer JR. Parkinson's disease.
Postgrad Med J.
1997;73:257-264.
8.
Shulman LM, Minagar A, Rabinstein A, et al.
The use of dopamine agonists in very elderly
patients with Parkinson's disease.
Mov Disord.
2000;15(4):661-668.
9.
Hughes A J, Daniel S E, Kilford L, et al.
Accuracy of clinical diagnosis of idiopathic PD:
a clinicopathological study of 100 cases.
J
Neurol Neurosurg Psychiatry. 1992;55:18184.
10.
Chaudhuri KR, Singh V, Agapito C, et al. Dual
agonist therapy with apomoprhine and cabergo-
line in advanced dyskinetic Parkinson's disease.
J
Neurol Neurosurg Psychiatry. 2000;69:422.
Abstract.
11.
Clarke CE, Deane KH. Cabergoline for lev-
odopa-induced complications in Parkinson's dis-
ease.
J Neurol. 2000;247(suppl 4):17-19.
12.
Rinne UK, Bracco F, Chouza C, et al. and the
PXDSOO9 Study Group. Cabergoline delays the
onset of motor complications resulted of double
blind levodopa controlled trial.
Drugs.
1998;55(suppl 1):23-30.
13.
Rinne UK. A 5 year double blind study with
cabergoline versus levodopa in the treatment of
early Parkinson's disease.
Park and Rel Disord.
1999;5(suppl):84.
14.
Frucht S, Rogers JD, Greene PE, et al. Falling
asleep at the wheel: motor vehicle mishaps in
persons taking pramipexole and ropinirole.
Neurology. 1999;52:1908-1910.
15.
Pal S, Bhattacharya KF, Agapito C, et al. A study
of excessive daytime sleepiness and its clinical
significance in three groups of Parkinson's dis-
ease patients taking pramipexole, cabergoline
and levodopa mono and combination therapy,
J
Neural Transm.
2001;108:71-77.
16.
Olanow CW, Schapira AV, Roth T. Waking up to
sleep episodes in Parkinson's disease.
Mov
Disord.
2000;15:212-215.
17.
Chaudhuri KR, al S, Brefel-Courbon C. Sleep
attacks or unintended sleep episodes occur with
dopamine agonists: is this a classs effect?
Drug
Safety.
2002;25:473-83.
18.
Sharma J. Do dopamine agonists stand alone?
Health and Ageing.
October 2002;25;S310-S316.
19.
Brecht HM. A comparison of dopamine agonists.
Aktuel Neurol.
1998;25;S310-S316.
Vol. 3, No. 4, Fall 2003 · The Journal of Applied Research
362






