|
The JOURNAL of APPLIED RESEARCH In Clinical and Experimental Therapeutics |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The Journal of Applied Research |
Genomics-Based
KGF-2 (Repifermin) and Its Receptors Function Effectively in Infected
Wounds
Martin C. Robson, MD*†
Terry E. Wright, MD*†
Francis Ko, BS*
Kevin M. Connolly, PhD‡
Wendy Halpern, DVM, PhD‡
Wyatt G. Payne, MD*†
*Institute for
Tissue Regeneration, Repair, and Rehabilitation, Bay Pines VA
†Department
of Surgery,
‡Human Genome Sciences, Inc.,
KEY WORDS: Repifermin, growth factors, receptors, bacteria, matrix metalloproteinases
ABSTRACT
Introduction: Cytokine growth factors have been ineffective in infected wounds (>105 bacteria/g of tissue) for several reasons. Bacteria degrade the polypeptides themselves, and this effect is accentuated in the presence of tissue cells when excessive matrix metalloproteinases are produced. Also, the growth factor receptors for several cytokines have been shown to have decreased expression in chronic wounds.
Materials and Methods: The effects of genomics-based KGF-2 (repifermin) on healing of infected wounds were examined in 3 experiments using a rodent model of a chronically infected (>105 bacteria/g of tissue) granulating wound. Serial wound area measurements were compared among animals treated with vehicle, 2 doses of KGF-2, and KGF-2 in combination with GM-CSF. Binding of biotinylated KGF-2 was compared with binding of a biotinylated irrelevant protein control in sections of both noninfected and infected granulation tissue. Serial biopsies of the wounds for quantitative bacteriology confirmed the wounds were infected throughout the experiments.
Results: In all 3 experiments, infected wounds healed faster when topically treated with KGF-2 (P <0.05). The effect was seen most dramatically toward the end of the wound closure curve when the wounds were 75% to 90% closed. Using GM-CSF in combination or in sequence with KGF-2 did not enhance the activity of KGF-2 alone. There was no clear decrease in KGF-2 binding in infected versus noninfected wounds. In contrast, sections incubated with the control-irrelevant protein were uniformly negative. Bacterial counts confirmed the wounds remained at a bacterial tissue level of >105 organisms/g.
Conclusion: Repifermin (genomics-based KGF-2) appears more robust than other reported growth factors in the presence of high levels of bacteria. This appears to be the result of its lesser degree of degradation in the presence of bacteria and tissue fibroblasts and the ability of its receptors to remain functional in infected tissue.
INTRODUCTION
Keratinocyte growth factor-2 (KGF-2), or repifermin, emerged from a genomics-based screening program in which 40 to 50 previously unknown proteins were evaluated by homology search.1 It is a member of the FGF family of mitogens. Its gene has been shown to be upregulated during wound healing, and it has been shown experimentally to accelerate several wound healing processes in experimental animal models.2,3 In a clinical trial of venous stasis ulcers, repifermin was demonstrated to be safe and to have activity in wound healing.1
Repifermin exerts
its effects through 2 receptors, the fibroblast growth factor receptor
(FGFR) 2iiib and the FGFR 1iiib.3–5 It has been shown to stimulate granulation
tissue formation and collagen deposition.6,7 Literature reports on its
effect on wound contraction are mixed. Jimenez et al.3 reported that
full-thickness excisional wounds in diabetic mice healed faster with
topically applied KGF-2, whereas Soler et al.8 were not able to demonstrate
more rapid contraction in a rat excisional wound model. However, the
doses of KGF-2 used in that report (1 mg and 15 mg KGF-2) might
have been insufficient.3
High levels of bacteria (>105 organisms/g of tissue) have been shown to inhibit all of the processes of wound healing.9 Bacteria alone have been demonstrated to cleave complex molecules and degrade and inactivate growth factors.10 Bacteria in the presence of tissue cells were demonstrated by Payne et al.10 to act synergistically to enhance growth factor degradation. Growth factor receptors have also been shown to be degraded in chronic contaminated wounds. Receptors for PDGF and TGF beta-1 have recently been reported to show decreased expression in chronic wounds.11,12 However, both KGF-2 receptors (FGFR 2iiib and KGFR 1iiib) have been found in all biopsies examined from human venous stasis ulcers.
In a rodent model of an infected granulating wound, bFGF required a dose 100-fold higher to effect wound contraction compared with a noninfected wound.13 When the vehicle was changed to a different vehicle to protect the growth factor molecule from bacterial degradation, the dose could be lowered 100-fold.14 In in vitro studies of bacterial degradation, all growth factors studied were degraded by bacteria alone. However, KGF-2 was not further degraded in the presence of tissue fibroblasts as were bFGF, GM-CSF, and TGF beta-2.10
Because KGF-2 (repifermin) was degraded to a lesser degree than other cytokine growth factors by bacteria in the presence of fibroblasts, and because the KGF-2 receptors remain present in chronic wounds, these experiments were performed to evaluate whether repifermin in normal clinical doses and the KGF-2 receptors could function effectively in infected wounds.
MATERIALS AND METHODS
Animal Model
Chronic infected granulating wounds were prepared as previously described.13–15 All experiments were conducted in accordance with the Animal Care and Use Committee guidelines of the Department of Veterans Affairs Medical Center, Bay Pines, Florida. Male Sprague-Dawley rats weighing 300 to 350 g were acclimatized for 1 week in our facility before use. Under intraperitoneal Nembutal anesthesia (35 mg/kg), the rat dorsum was shaved and depilated. A full-thickness dorsal burn measuring 30 cm2 was created by immersion in boiling water. The burns were inoculated with 5 ¥ 108 Escherichia coli (ATCC #25922 American Type Culture Collection, Rockville, MD) after the rats had been allowed to cool for 15 minutes. Bacteria were obtained from fresh 18-hour broth cultures and inoculum size was confirmed by back-plating.
Animals were individually caged and given food and water ad libitum. Five days after burning, the eschar was excised from anesthetized animals resulting in a chronic granulating wound. Histologic characterization of the wound has previously been shown to be comparable to a human chronic granulating wound.16
All wounds were left exposed. Any dried exudate was atraumatically removed before application of test treatments, before wound biopsies, or before wound measurements. Wounds were biopsied for quantitative bacteriology at initial eschar debridement and 3, 6, and 9 days after escharectomy to exclude superinfection and to confirm bacterial levels in the infected wounds.17 In one group of animals noninfected wounds were compared with infected wounds and biopsies were performed 3 and 15 days after escharectomy to test for the presence of functional KGF-2 receptors. Every 48 hours the outlines of the wounds were traced onto acetate sheets, and area calculations were performed using computerized digital planimetry (Sigma Scan, Jandel Scientific, Corte Madeira, CA). Care was taken to record the advancing full-thickness margin as well as the edge of any advancing epithelium. All animals were weighed on a weekly basis.
The animals were sacrificed by Nembutal overdose and bilateral thoracotomies when the wound completely healed, or healed to less than 10% of its original area. Hayward et al.13 demonstrated that measurement of very small wounds by manual tracing introduced significant systematic error and found that wounds followed past this point remained static for prolonged periods of time.
Treatment Groups
A series of 3 different experiments were performed. In the first experiment, 40 rats were divided into 4 groups of 10 rats each. Group 1 served as an uninfected control for the purpose of assessing the functional receptor for KGF-2. Group 2 was infected with 108 colony-forming units of E. coli, and after their eschars were excised, they were treated with the same topical vehicle as that used for the KGF-2 dilutions (vehicle controls) daily. Group 3 had their infected wounds treated with 60 mg/cm2 KGF-2 (Human Genome Sciences, Rockville, MD) per day after escharectomy. Group 4 had their infected wounds treated with 100 mg/cm2 repifermin (KGF-2) per day after escharectomy. On days 3 and 15 after escharectomy, biopsies from animals in groups 1 and 2 were placed in 10% formalin for later analysis of the functional KGF-2 receptors.
In the second experiment 10 animals were divided into 2 groups of 5.
Group 1 was inoculated with 108 E. coli and treated with the KGF-2 vehicle per day after escharectomy. Group 2 was inoculated but treated with 60 mg/cm2 repifermin per day after escharectomy.
In
the third experiment, attempts were made to evaluate a mixture of cytokines
including KGF-2 or sequential use of a proinflammatory cytokine growth
factor (GM-CSF) followed by repifermin. Twenty-four rats were divided
into 4 groups of 6 animals each. Animals in group 1 were treated with
GM-CSF (Schering-Plough Research, Kenilworth, NJ) (10 mg/cm2
per day for 10 days after escharectomy). Animals in group 2 were treated
with KGF-2 (100 mg/cm2) per
day for 10 days after escharectomy. Animals in group 3 were treated
daily with a cocktail of GM-CSF
(10 mg/cm2) and KGF-2 (100 mg/cm2) for 10 days after escharectomy. Finally,
animals in group 4 were treated with GM-CSF (10 mg/cm2) per day for 5 days after escharectomy,
followed by treatment with KGF-2 (100 mg/cm2) per day for 5 days.
Quantitative Bacteriology
On the day of eschar debridement and on days 3, 6, and 9 after escharectomy, biopsies were obtained after cleansing the wound surface with 70% isopropyl alcohol to exclude surface contamination. The biopsies were weighed, flamed, and homogenized after being diluted 1:10 weight to volume with thioglycolate. Serial tube dilutions were prepared and then backplated onto selective media. Bacterial counts were completed after 48 hours incubation and expressed as colony-forming units (CFU) per gram of tissue.17
KGF-2 Functional Receptor Analysis
The ability of biotinylated KGF-2 protein to bind to the uninfected and infected wound biopsies (groups 1 and 2 of the first experiment) was compared with the ability of an irrelevant protein, biotinylated B-lymphocyte stimulating factor (BLyS), to bind to the same wound biopsies. Binding of KGF-2, but not BlyS, is evidence of functioning KGF-2 receptors in the test biopsy.
Formalin-fixed paraffin-extracted tissue blocks were sectioned at 5 mm using a Microm microtome and air-dried overnight. Slides were heated in a 65oC oven for 30 minutes, deparaffinized, then treated to remove endogenous peroxidase in 0.3% H2O2 in methanol (Fisher Scientific, Pittsburgh, PA) for 30 minutes. Slides were washed in 0.01 M phosphate-buffered saline (PBS) and then treated with 0.025% protease VIII in 0.02 M Tris/0.002 M CaCl2 (Sigma, St. Louis, MO) for 20 minutes at room temperature. They were then washed in Tris/0.001 M glycine (Sigma) and mounted in Sequenza staining chambers.
Nonspecific binding was blocked by incubating all slides in PPS/bovine serum albumin (BSA; Sigma) for 30 minutes. Biotinylated KGF-2 (Human Genome Sciences, Rockville, MD) and biotinylated BLyS (Human Genome Sciences) were diluted to 10 mg/mL in PBS/BSA and sections incubated overnight at 4˚C. Sections were washed with PBS and Tween-20 (PBS-T; Fisher Scientific, Pittsburgh, PA) followed by 0.01 M PBS.
Binding was detected using the Streptavidin-HRP detection kit (Vector Laboratories, Burlingame, CA) followed by application of diaminobenzidine (DAB; Sigma) for 1 minute, coverslipped, and examined microscopically.
Statistical Analysis
Serial area measurements of the rat-infected granulating wounds were plotted against time. For each animal’s data, a Gompertz equation was fitted (typical r2 = 0.85).15,18 Using this curve, the wound half-life was estimated. Comparison between groups were performed using life table analysis and Wilcoxon rank test.18 These statistical analyses were performed using the SAS (SAS/STAT Guide for Personal Computers, version 6, Cary, NC, 1987, p1028) and BMDP (BMDP Statistical Software Manual, BMDP Statistical Software, Inc., Los Angeles, CA, 1998) packages on a personal computer.
RESULTS
Effect of Repifermin (KGF-2) on
Wound Area
In experiment 1, repifermin improved the rate of healing by contraction at both 60 mg/cm2 and 100 mg/cm2 (Fig. 1). This difference was statistically significant when the wounds were 50% closed and 90% closed (P <0.05). In the second experiment, 60 mg/cm2 KGF-2 per day again was effective at speeding healing by contraction. At 75% closure and 90% closure, the differences were significant (P = 0.033 and P = 0.006) (Fig. 2). Using GM-CSF in combination or in sequence with KGF-2 was not as effective as using KGF-2 (100 mg/cm2) alone in the third experiment (Fig. 3). The group treated with KGF-2 alone closed statistically faster at 75% closure than the GM-CSF treated group (P = <0.001), the GM-CSF/KGF-2 mixture group (P = 0.006), and the sequential GM-CSF/KGF-2 group (P = 0.004). These significant differences remained at 90% wound closure (P <0.001).
Quantitative Bacteriology
Biopsies of the noninfected group in experiment 1 showed <105 organisms/g of tissue on all biopsy days (Table 1). Infected animals treated with KGF-2 in all 3 experiments showed consistent tissue levels of bacteria greater than 105 E. coli CFU per gram of tissue (Table 1). The bacterial count decreased only when GM-CSF was present.
Body Weights
There was an equivalent gain in body weight among all
groups during the various periods of study in all 3 experiments with
no significant variation among the groups.
Functional KGF-2 Receptors
There was no clear decrease in KGF-2 binding in infected
versus noninfected wounds (groups 1 and 2) in experiment 1. Staining
for biotinylated KGF-2 occurred in the epidermis, when present in the
healing wounds, and in granulation tissue. It was strongest in mononuclear
tissue macrophages within the granulation tissue. In contrast, sections
inoculated with the control irrelevant protein (biotinylated BLyS) were
uniformly negative. The staining observed in sections incubated with
KGF-2, but not with the irrelevant protein control, suggests a low level
of diffuse receptor expression in both infected and noninfected granulating
wounds.
DISCUSSION
Prudent care of contaminated chronic wounds dictates that wounds be placed into bacterial balance (105 or fewer bacteria/g of tissue) before treatment with polypeptide cytokine growth factors.19,20 High levels of bacteria inhibit each of the processes of wound healing and are known to decrease growth factor and receptor levels in infected wounds.9–12 In recent clinical trials of repifermin (KGF-2) to treat venous stasis ulcers, patients were required to have wounds containing less than 1 ¥ 106 organisms/g of tissue and no beta-hemolytic streptococci before trial enrollment.1
In the established model of an infected granulating wound model used in these experiments, both bFGF and PDGF-BB required large doses of cytokines to overcome the inhibition of contraction caused by the bacteria unless a vehicle was used to protect the molecules13,14 (Robson MC, unpublished data). These doses were 100 times those required in clinical trials when the wounds contained 105 or fewer bacteria/g of tissue.21,22 Only when a proinflammatory cytokine such as GM-CSF that lowered the bacterial count in the granulation tissue was used, were nonelevated doses of the cytokine effective in this model.23
In the present study, clinically studied doses of repifermin (60 mg/cm2 and/or 100 mg/cm2) were effective at accelerating healing of the infected wounds1 (Figs. 1, 2, and 3). This could be the result of the fact that the bacteria in the infected wounds did not destroy the KGF-2 to the extent that they do other growth factors and/or that the bacteria did not render the receptors in the wound ineffective. Payne et al.10 showed that bacteria alone degraded KGF-2 to approximately the same degree as other growth factors tested. However, they showed that when bacteria were in the presence of tissue fibroblasts like they would be in the infected rodent wound, KGF-2 was degraded statistically less than bFGF, GM-CSF, or TGF beta-2.10 In that report, the increased degree of degradation by bacteria in the presence of fibroblasts was most marked for GM-CSF, which might explain GM-CSF’s relatively poorer performance in our third experiment.
The functional KGF-2 receptors also appeared to remain effective in the presence of high bacterial levels. Although the staining demonstrated after incubation with KGF-2 could possibly have been the result of nonspecific binding, it is more likely the result of a low level of diffuse receptor expression. This is especially likely to be the case because sections of each biopsy were uniformly negative when incubated with a biotinylated irrelevant protein. Both KGFR-2iiib and KGFR-1iiib transcripts have been demonstrated by RT-PCR to be present in biopsies from human venous stasis ulcers (Olsen HS, Garcia AD, unpublished data). However, their mere presence did not confirm they were functional. In experiment 1 we have shown that the receptors are present and functional by their ability to bind the biotinylated KGF-2.
It is interesting
that the largest increases in closure rate occurred toward the end of
the contraction curves (Figs. 1, 2, and 3). This portion of the curve
is more affected by keratinocytes than by fibroblasts.15 Repifermin
(KGF-2) has shown no mitogenic activity for cultured fibroblasts, and
was not effective in increasing contraction in a different model of
excisional wounds.3,24 These data suggest that possibly repifermin might
be useful in combination or sequence with a cytokine such as bFGF that
affects contraction directly by its effect on fibroblasts.
The model used in
these experiments is extreme in that the levels of bacteria are greater
than 105 bacteria/g of tissue. As has been stated, it is not desirable
to apply exogenous cytokines to heavily infected wounds. However, these
data do suggest that repifermin and its receptors might be more robust
than other reported cytokine growth factors in contaminated clinical
chronic wounds that have been brought into bacterial equilibrium.
REFERENCES
1. Robson MC, Phillips TJ, Falanga V, et al: Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Rep Regen 9:347–352, 2001.
2. Tagashira S, Harade H, Katsumata T, Itoh N, Nakatuka M: Cloning of mouse FGF 10 and up-regulation of its gene during wound healing. Gene 197:399–404, 1997.
3. Soler PM, Wright TE, Smith PD, et al: In vivo characterization of keratinocyte growth factor-2 as potential wound healing agent. Wound Rep Regen 7:172–178, 1999.
4. Igarashi M, Finch PW, Aaronson SA: Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem 273:13230–13235, 1998.
5. Jimenez PA, Greenwalt D, Mendrick DL, et al: Keratinocyte growth factor-2. In: Narula SK, Coffman R, Eds. New cytokines as potential drugs. Basel, Switzerland: Birhauser Verlag; 2000:101–119.
6. Duan DR, Jimenez PA, Gruber JR, et al: Identification of keratinocyte growth factor-2 that promotes cutaneous wound repair. Wound Rep Regen 5:A111, 1997.
7. Xia YP, Zhao Y, Marcus J, et al: Effects of keratinocyte growth factor-2 (KGF-2) on wound healing in an ischemia-impaired rabbit ear model and on scar formation. J Pathol 188:431–438, 1999.
8. Jimenez P, Telsika M, Liu B, Judis K, Rampy M, Antonaccia M: Keratinocyte growth factor-2: A wound healing agent with epidermal and dermal activity. Wound Rep Regen 5:A115, 1997.
9. Robson MC, Stenberg BD, Heggers JP: Wound healing alterations caused by infection. Clin Plast Surg 17:485–492, 1990.
10. Payne WG, Wright TE, Ko F, Wheeler C, Wang X, Robson MC: Bacterial degradation of growth factors. J Appl Res (in press).
11. Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Cohen IK: Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Rep Regen 5:23–32, 1997.
12. Hassan A, Murata H, Fallabella A, et al: Dermal fibroblasts from venous ulcers are unresponsive to the action of transforming growth factor beta-1. J Dermatol Sci 16:59–66, 1997.
13. Hayward P, Hokanson J, Heggers J, et al: Fibroblast growth factor reverses the bacterial retardation of wound contraction. Am J Surg 163:288–293, 1992.
14. Kuhn MA, Page L, Nguyen K, Wang X, Wachtel TL, Robson MC: Basic fibroblast growth factor in a carboxymethylcellulose vehicle reverses the bacterial retardation of wound contraction. Wounds 13:73–80, 2001.
15. Hayward PG, Robson MC: Animal models of wound contraction. In: Barbal A, Caldwell M, Eaglstein W, et al., Eds. Clinical and experimental approaches to dermal and epidermal repair: normal and chronic wounds. New York: Alan R. Liss; 1991:301–312.
16. Robson MC, Edstrom LE, Krizek TJ, Groskin MG: The efficacy of systemic antibiotics in the treatment of granulating wounds. J Surg Res 16:299–306, 1984.
17. Heggers JP: Variations on a theme. In: Heggers JP, Robson MC, Eds. Quantitative bacteriology: Its role in the armamentarium of the surgeon. Boca Raton, FL: CRC Press; 1991:15–23.
18. Hokanson JA, Hayward PG, Carney OH, Phillips LG, Robson MC: A mathematical model for the analysis of experimental wound healing data. Wounds 3:213–220, 1992.
19. Robson MC, Mustoe TA, Hunt TK: The future of recombinant growth factors in wound healing. Am J Surg 176(suppl):80s–82s, 1998.
20. Robson MC: Wound infection: A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 77:637–650, 1997.
21. Robson MC, Phillips LG, Thomason A, et al: Recombinant human platelet-derived growth factor-BB for the treatment of chronic pressure ulcers. Ann Plast Surg 29:193–201, 1992.
22. Robson MC, Phillips LG, Lawrence WT, et al: The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores. Ann Surg 216:401–408, 1992.
23. Kucukcelebei A, Carp SS, Hayward PG, et al: Granulocyte-macrophage colony stimulating factor reverses the inhibition of wound contraction caused by bacterial contamination. Wounds 4:241–247, 1992.
24.
Emoto H, Tagashira S, Mahai M, et al: Structure and expression
of human fibroblast growth factor-10. J Biol Chem 272:23191–23194, 1997.
Figure 1. Healing of KGF-2—treated infected wounds
compared with vehicle-treated infected wounds shows accelerated healing
when the wounds are 50% closed and 90% closed (P<0.05). KGF-2 was
effective both at the 60 and 100 µg/cm2 doses.
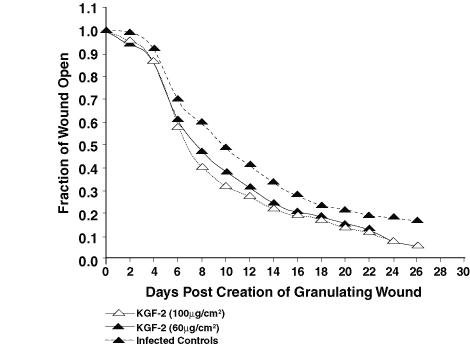
Figure 2. In experiment 2, the KGF-2 (60 _g/cm2)-treated
wounds healed statistically faster than non-treated wounds at 75% closure
and 90% closure (P=0.033 and P=0.006).
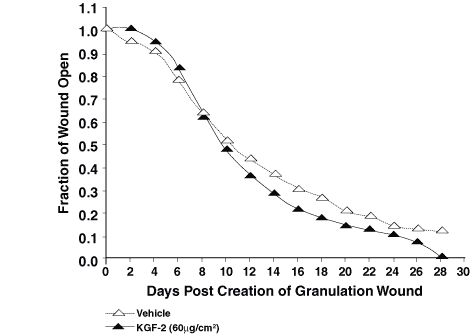
Figure 3. The KGF-2 alone—treated wounds closed
statistically faster at 75% closure than the GM-CSF—treated wounds (P<0.001),
the GM-CSF/KGF-2 mixture—treated wounds (P=0.006), and the sequential
GM-CSF/KGF-2 treated wounds (P=0.004). These significant differences
remained at 90% wound closure (P=0.001).
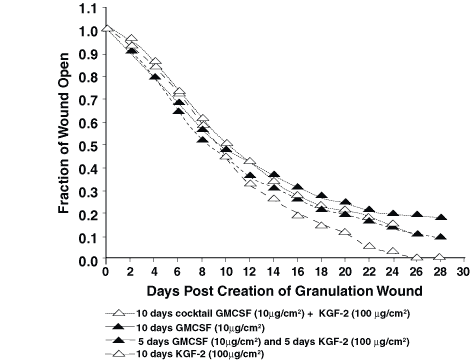
Table 1. Quantitative Bacteriology Results for
Wound Biopsies Collected on Day of Eschar Removal (0) and on Day 3,
6, and 9 After Eschar Removal*
Group Escharotomy Day 0 Day 3 Day 6 Day 9
1-Exp. 1 (uninfected) <105 <102 <102 <102
2-Exp. 1 (infected controls) 9.3 ¥ 107 1.8 ¥ 107 2.0 ¥ 107 3.6 ¥ 107
3-Exp. 1 (KGF-2 60 µg/cm2) 4.3 ¥ 107 3.3 ¥ 107 1.7 ¥ 107 8.8 ¥ 106
4-Exp. 1 (KGF-2 100 µg/cm2) 6.0 ¥ 107 1.7 ¥ 107 2.4 ¥ 107 8.0 ¥ 106
1-Exp. 2 (infected controls) 8.0 ¥ 107 6.0 ¥ 107 2.0 ¥ 107 5.6 ¥ 106
2-Exp. 2 (KGF-2 60 µg/cm2) 8.2 ¥ 107 5.0 ¥ 107 9.9 ¥ 106 2.9 ¥ 105
1-Exp. 3 (GM-CSF 10 µg/cm2) 7.4 ¥ 107 5.4 ¥ 105 4.2 ¥ 105 1.0 ¥ 105
2-Exp. 3 (KGF-2 100 µg/cm2) 9.3 ¥ 107 5.8 ¥ 107 3.6 ¥ 107 9.4 ¥ 106
*Results are mean counts for treatment groups expressed in colony forming units per gram of tissue.
©2000-2013. All Rights Reserved. Veterinary Solutions LLC
2Checkout.com is an authorized retailer for The
Journal of Applied Research