|
The JOURNAL of APPLIED RESEARCH In Clinical and Experimental Therapeutics |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The Journal of Applied Research |
The Relationship
Between Pneumonia and Gastric Colonization in Surgical Intensive-Care
Unit Patients
B. H. Uçan, MD*
F. Begendik†
M. O˘guz,
MD‡
*Department of General Surgery,
†Department
of Microbiology,
‡Department
of General Surgery,
KEY WORDS: nosocomial pneumonia, intensive-care unit, gastrointestinal colonization
ABSTRACT
Objective: To evaluate the correlation of intensive-care unit (ICU)-acquired pneumonia and gastric colonization, and the factors affecting gastric colonization in surgical ICU patients.
Design: Prospective, clinical study.
Setting: Surgical ICU of Gazi University, Faculty of Medicine, Department of General Surgery.
Patients: Sixty-seven adult surgical ICU patients who did not have pneumonia on admission and would have nasogastric (NG) tubing at least for 48 hours were evaluated between October 1996 and April 1998.
Method: Patients who were approved not to have pneumonia by physical examination, chest x-ray, white blood cell count, and sputum culture on admission were included in the study. NG tube aspirate and sputum samples for microbiologic studies, chest x-ray, and white blood cell count were obtained every other day and additionaly if there were symptoms or signs of infection.
Results: Seventeen
of the 67 patients (25.37%) had pneumonia in the period of ICU stay.
In total, 122 NG tube aspirate and 122 sputum samples were obtained
simultaneously. Of these 122 NG tube aspirate and sputum cultures,
85 couples of samples (69.67%) had NG tube aspirate culture (–)/sputum
culture (–), 20 couples of samples (16.40%) NG tube aspirate culture
(+)/sputum culture (+) (the same bacteria was isolated). Pneumonia
in 13 of the 17 patients who had gastric colonization were the result
of the same bacteria as isolated from the patient’s gastric fluid.
Conclusion: Based on the findings, gastric colonization is a serious predisposing factor for pneumonia. When both the dual-positive and the dual-negative culture results are taken into account, a strong positive correlation of the nasogastric tube aspirate and sputum cultures can be seen. Therefore, it is recommended to obtain the upper gastrointestinal (GI) fluid from indwelling gastric or enteric tubes periodically for microbiologic studies to predict the possible bacteria that could lead to pneumonia and moreover to take preventive or treatment measures.
INTRODUCTION
With the intensive-care units (ICUs) being in use to treat physiologically unstable patients in the same unit, it has been realised that nosocomial infections among ICU patients are much more common and seen two- to fivefold higher than in non-ICU patients.1 A number of studies, done to investigate the reasons and sources of these infections, have suggested that the ICU staff,2 contaminated cathaters and equipment,3 intensive surgical interventions, and the medications could be responsible. However, in the past two decades, it was shown that the reservoir for most of these infections was the patients’ own flora, and also the upper gastrointestinal (GI) system could act as a reservoir when pathologic colonization occurred.4,5 Thus, attention has been directed onto the proximal GI system that is normally sterile, the factors that derange homeostasis and lead to pathologic colonization of the proximal GI system, and the interaction between pathologic colonization of gut and nosocomial infections. It has been reported that among the nosocomial infections, pneumonia had the strongest correlation with gastric colonization in terms of the microorganisms isolated.6,7
The aim of this study was to determine the relevance of simultaneous sputum and nasogastric (NG) aspirate cultures in case of nosocomial pneumonia and upper gut bacterial colonization, and to investigate the factors that predispose to this colonization and the clinical use of NG tube aspirate culture.
PATIENTS AND METHODS
The study protocol was ratified by the Human Ethics Committee of Gazi University Hospital. Sixty-seven patients with ages ranging between 22 and 88 years (mean, 57.2 y) admitted to the surgical ICU from October 1996 to April 1998 were included in this study. Patients with pneumonia on admission were excluded. In an attempt to quantify the severity of illness, APACHE II scores of patients on admission were assigned.8 NG tubing was applied to all of the patients, and correct placement was confirmed by auscultation and a radiograph. Ranitidine at a dosage of 100 mg every 8 hours administered intravenously was given to the patients with a history of peptic ulcer disease or under the risk of GI bleeding.9
NG tube aspirate and sputum samples, posterior-anterior chest x-ray, white blood cell count, and physical examination were carried out at every 2 days periodically. Gastric fluid samples were obtained from indwelling NG tubes. After removing the initial 5 mL, the next 10 mL of fluid in the tube were aspirated into a sterile syringe and immediately transported to the laboratory for processing. From the patients with endotracheal intubation, sputum samples were obtained by way of bronchoalveoler lavage aspiration. One hundred microliters of each dilution was plated on blood agar and MacConkey agar for aerobes, and on blood agar and phenylethyl alcohol agar for anaerobes. Aerobic plates were incubated for 24 hours at 37˚C and anaerobic plates for 48 hours at 37˚C. Colonies were then enumerated and species were identified by using the API 20E (BioMerieux Vitek, Inc., Hazelwood, MO) for identification of gram-negatives and the standard techniques for gram-positives. Candida organisms were identified by growth on blood agar and by morphology in wet preparation. The diagnosis of colonization was made if more than 103 colony-forming units per milliliter were found. Pneumonia was diagnosed according to the presence of those criteria: fever >38˚C, increase in blood leukocyte count (an increase of >3 ¥ 109 WBC/L), new and progressive infiltrate on chest radiograph, and positive sputum culture result. The results of 122 NG tube aspirate and sputum cultures obtained from 67 patients were analyzed.
Statıstıcs
Results were compared using the chi-square statistics with Fisher exact test and considered to be significant for values of P <0.05.
RESULTS
The mean age of the patients was 57.2 years (range, 22–88 y) with a male/female ratio of 44/23. Among the 67 patients, 47 were the patients undergoing major surgical procedures and 14 were trauma patients (Table 1). Two patients had severe acute pancreatitis and four patients had subacute cholecystitis. The latter six patients were treated medically, but, because of their high APACHE II scores, they were hospitalized in the surgical ICU. Five of the 14 trauma patients, 11 of the 47 patients undergoing major surgical procedures, and 1 of the 6 patients with medical treatment were diagnosed as having pneumonia. The pneumonia incidences of these groups were 35.71%, 23.40%, and 16.40%, respectively. In total, 122 simultaneous NG tube aspirate and sputum samples were obtained for microbiologic studies from 67 patients. The results of these cultures are listed in Table 2. In eighty-five couples of samples (69.67%) the culture results were as NG tube aspirate culture (–)/sputum culture (–), on the other hand, in 20 couples of samples (16.40%) the cultures resulted as NG tube aspirate culture (+)/sputum culture (+) (isolated bacterias were the same for each couple).
Nutritional support was given to 23 patients in the form of total parenteral nutrition (TPN) + partial enteral nutrition (PEN) in 14, whereas in the form TPN only in 9. The frequencies of gastric colonization in the TPN group, in the TPN+PEN group, and in the group without nutritional support (n = 44) were 88.89%, 50.00%, and 4.55% and the frequencies of pneumonia in these groups were 88.89%, 42.86%, and 6.82%, respectively.
The APACHE II scores and pneumonia frequencies are shown in Figure 1. As summarized in Figure 2, pneumonia incidences were in close correlation with the APACHE II scores. For example, although there was no pneumonia in the 0–4 APACHE II group, if the APACHE II score was 25 or more, all of the patients would develop pneumonia.
Thirty-one of the 122 NG tube aspirate cultures were positive (Table 3). The most colonizing species were coagulase (+) Staphylococcus (n = 10), Klebsiella (n = 8), and Pseudomonas (n = 4). Thirty of the 122 sputum cultures were positive (Table 4), and the most colonizing species were coagulase (+) Staphylococcus (n = 11), Pseudomonas (n = 8), and Klebsiella (n = 5).
Thirty patients had ranitidine for prophylaxis of stress ulceration and/or upper GI bleeding. Whereas the gastric colonization incidence of ranitidine group was 46.42% (n = 14), in the group not treated with ranitidine or any other anti-ulcer agent, the incidence was 8.11% (n = 3) (Fig. 2). The difference was statistically significant (P <0.0008).
Seventeen of the 67 patients had pneumonia; the pneumonia incidence was 25.37% in the study period. The microorganisms isolated from both the NG tube aspirate and sputum samples of 13 of the 17 pneumonia cases were the same. Gastric colonization was present in 17 of the 67 patients (Table 4). Among the 17 patients who had positive NG tube aspirate cultures, the incidence of pneumonia was 82.35% (n = 14). The NG tube aspirate cultures of the remaining 50 patients were negative and pneumonia was seen only in three of these patients (6.0%). The difference between these two group was statistically significant (P < 0.0001).
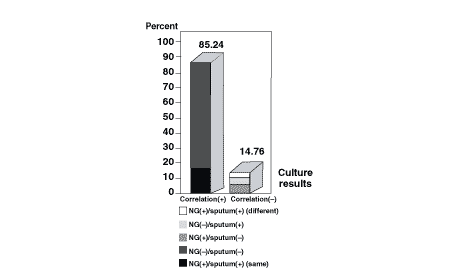
As summarized in Figure 3, 85 couples of the NG tube aspirate and sputum cultures resulted as NG tube aspirate culture (–)/sputum culture (–), and 20 couples of NG tube aspirate and sputum cultures resulted as NG tube aspirate culture (+)/sputum culture (+) (the same bacterias were isolated in any one of these couples). Thus, if both-positive and both-negative culture results were considered together, the correlation of the NG tube aspirate cultures with the sputum cultures was 85.24%.
DISCUSSION
Stomach and proximal GI tract are sterile or occasionally populated with a relatively avirulent flora, including Lactobacilli and Streptococci, in healthy individuals.10,11 There are a number of factors, including gastric acidity,2 normal intestinal motility,12 bile salt,13 mucosal IgA,14 and defensins from Paneth cells,15 maintaining the relative sterility of the upper GI tract. Furthermore, enteral feeding stimulates gastric acid secretion, enhances bile flow and intestinal motility, induces cholecystokinin and secretin release, and regulates the production of IgA from intestinal mucosa.16 It was demonstrated that the institution of enteral nutrition, as soon in the postoperative period as possible, reduced the rates of nosocomial infections.17 In our study, nutritional support was given to 23 of the patients. While 9 of the 23 patients received TPN, 14 patients received TPN + PEN. Pneumonia and gastric colonization developed in 8 (88.89%) of the patients receiving TPN. Besides in the TPN + PEN group (n = 14), gastric colonization developed in 7 (50%) of the patients and pneumonia developed in 6 (42.86%) of the patients; the difference between these two groups were not significant statistically. Nevertheless, the difference between the groups without any form of nutritional support and with TPN or TPN + PEN was significant (P < 0.0008). Therefore, it looks like as if the nutritional support has enhanced the rate of nosocomial pneumonia. However, it is important that the patients in need of nutritional support have had one or more of the risk factors for pneumonia such as a high APACHE II score, mechanical ventilation, malignancy, major trauma, or a major surgical intervention.
Ranitidine, which is widely used for prophylaxis of stress ulcers and GI bleeding, has two important effects on gastric homeostasis. Although ıt is decreases the rates of stress ulcer and upper GI bleeding, on the other hand it predisposes the stomach for pathologic colonization by increasing the gastric pH over 4. Eddleston et al.18 investigated the advantages and disadvantages of ranitidine and sucralfate, a cytoprotective agent with little or no effect on gastric pH, and reported that, despite the fact that the protective effects of these two agents against ulceration and bleeding were similar, the alkalinization of the gastric juice with ranitidine was greater than with sucralfate and thus, pathologic gastric colonization and pneumonia were more common in the ranitidine group. Some of the other authors also have reported similar findings.5,19,20 The gastric acidity is much more important for aerobic Gram-negative bacilli because the consequence of the alkalinization of gastric pH to more than 4 is known to result in the colonization of stomach by aerobic Gram-negative bacilli and yeasts.5 This phenomenon can progress to nasopharyngeal and later tracheal colonization,4,5,19 and also to pneumonia.21 In our study, gastric colonization developed in 14 (46.67%) of the 30 patients who had ranitidine prophylaxis. However, gastric colonization developed only in 3 (8.10%) of the 37 patients who did not have any prophylactic treatment against ulcer and/or bleeding. The difference between these two groups was statistically significant (P < 0.0008). In 17 of the 67 patients who developed gastric colonization, pneumonia incidence was significantly higher than in the remaining 50 patients who did not develop gastric colonization (P < 0.0001). The use of ranitidine has increased the gastric colonization; therefore, it seems to be an important predisposing factor for pneumonia. Supporting this are the results of the study by Blair et al.22 reporting that the decontamination of the digestive system by systemic antibiotics has reduced the rates of nosocomial infections and mortality. There are a number of other studies with similar results,23,24 although the efficacy of this strategy is in question.25
The APACHE II score has been used to determine the quantitative severity of illness in a number of studies; it has been reported that, while the APACHE II score is increasing, the mortality and morbidity also increase.7,8,26 In our study, it was found that a strong correlation was present between the APACHE II score and the pneumonia incidence. The incidence of pneumonia was significantly higher, especially if the APACHE II score was 15 or more (P < 0.0008). Morover, all of the patients whose APACHE II scores were 25 or more developed pneumonia. Therefore, we agree with these studies in that the APACHE II score has a reliable predictive value for morbidity and mortality.
The most colonizing species in the stomach were coagulase (+) Staphylococcus (n = 10), Klebsiella (n = 8), and Pseudomonas (n = 4). The most frequent microorganisms isolated from the sputum samples were coagulase (+) Staphylococcus (n = 11), Pseudomonas (n = 8), and Klebsiella (n = 5). All of the 4 (100%) patients with Pseudomonas-positive NG tube aspirates developed Pseudomonas pneumonia, whereas 8 (80%) of 10 patients with gastric colonization of coagulase (+) Staphylococcus and 4 (50%) of 8 patients with gastric colonization of Klebsiella developed pneumonia with the same microorganism that colonized in the stomach. Thus, Pseudomonas seemed to be the most virulent bacteria leading to pneumonia among the microorganisms isolated from the gastric fluid.
There are a few hypotheses explaining the pathophysiology of the phenomenon that the pathologic gastric colonization might lead to nosocomial infections. ICU-acquired pneumonia resulting from subclinical aspirations of contaminated gastric secretions is a well-documented phenomenon,4,27 which is thought by many authors to be the most important cause of pneumonia in ICUs.28 In addition, subclinical aspiration in conjunction with NG tubes has been reported to be a risk factor for the development of ICU-acquired pneumonia.29 Another hypothesis is that the retrograde bacterial colonization from stomach to oropharynx and trachea causes pneumonia.18 On the other hand, the occurrence of the other nosocomial infections such as urinary tract infections and bacteremia with organisms simultaneously present in the gut has suggested that bacterial translocation might be a factor of importance in the pathogenesis of ICU-acquired infections.6
No matter whether the relationship between gut colonization and systemic infection reflects a cause-effect relationship or simply a coincidence, it is apparent that the microbial milieu of the critically ill patient is well reflected in the gut in case of its colonization that evolves during the ICU stay.6. Our findings, which suggest that pathologic gastric colonization might be one of the most important risk factors for pneumonia, also support this fact. A strong correlation of NG tube aspirate cultures with sputum cultures has been shown in this study. By noting that sampling of the gastric juice is relatively noninvasive and easy to perform, it is recommended to obtain upper GI fluid for microbial evaluation by way of indwelling gastric or enteric tubes periodically for microbiologic studies to predict the cause of pneumonia that can arise, and furthermore to plan preventive measures at least for high-risk patients.
REFERENCES
1. Maki DG: Risk factors for nosocomial infection in intensive care: ‘devices vs nature’ and goals for the next decade. Arch Intern Med 149:30, 1989.
2. Klein BS, Perloff WH, Maki DG: Reduction of nosocomial infection during pediatric intensive care by protective isolation. N Engl J Med 320: 1714, 1989.
3. Maki DG: Risk factors for nosocomial infections in intensive care units. Intens Care Med 16(suppl 3):S197, 1990.
4. du Moulin GC, Hedley-White J, Paterson DG, et al: Aspiration of gastric bacteria in antiacid-treated patients: a frequent cause of postoperative colonisation of the airway. Lancet 1:242, 1982.
5. Garvey BM, McCambley JA, Tuxen DV: Effects of gastric alkalization on bacterial colonization in critically ill patients. Crit Care Med 17:211, 1989.
6. Marshall JC, Christou NV, Meakıns JL: The gastrointestinal tract: the ‘undrained abscess’ of multiple organ failure. Ann Surg 218:111, 1993.
7. Craven DE, Kunches LM, Lichtenberg DA, et al: Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med 148:1161, 1988.
8. Knaus WA, Draper EA, Wagner DP, et al: APACHE II: a severity of disease classification system. Crit Care Med 13:818, 1985.
9. Cook DJ, Fuller HD, Guyatt GH, et al: Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med 330:377, 1994.
10. Gorbach SL, Plaut AG, Nahas L, et al: Studies of intestinal microflora. II. Microorganisms of the small intestine and their relations to oral and fecal flora. Gastroenterol 53:856, 1967.
11. Finegold SM, Sutter VL, Mathisen GE: Normal indigenous intestinal flora. In: Hentges DJ, ed. Human Intestinal Microflora in Health and Disease. New York: Academic Press; 1983:3–31.
12. Lee A:
Neglected niches: the microbial ecology of gastrointestinal tract.
Adv Microb Ecol 8:115, 1985.
13. Floch MH, Binder HJ, Filburn B, et al: The effect of bile acids on intestinal microflora. Am J Clin Nutr 25:1418, 1972.
14. McLaughlin GA, Hede JE, Temple JG, et al: The role of IgA in the prevention of bacterial colonization of the jejunum in the vagotomized subject. Br J Surg 65:435, 1978.
15. Eisenhauer PB, Harwing SSL, Lehrer RI: Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immunol 60:3556, 1992.
16. Shah PC, Freier S, Park BH, et al: Pancreozymin and secretin enhance duodenal fluid antibody levels to cow’s milk proteins. Gastroenterol 83:881, 1982.
17. Moore FA, Feliciano DV, Andrassy RJ, et al: Early enteral feeding, compared with parenteral, reduces postoperative septic complications. Ann Surg 216:172, 1992.
18. Eddleston JM, Vohra A, Scott P, et al: A comparison of the frequency of stress ulceration and secondary pneumonia in sucralfate or ranitidine treated intensive care unit patients. Crit Care Med 19:1491, 1991.
19. Ruddel W, Axon A, Findlay J, et al: Effect of cimetidine on the gastric bacterial flora. Lancet ii:672, 1980.
20. Atherton S, White D: The stomach as a source of bacteria colonising the respiratory tract during artificial ventilation. Lancet ii:968, 1978.
21. Tryba M: Risk of acute stress bleeding and nosocomial pneumonia in ventilated intensive care unit patients: sucralfate versus antacids. Am J Med 22(suppl 140):7, 1987.
22. Blair P, Rowlands BJ, Lowry K, et al: Selective decontamination of the digestive tract: a stratified, randomized, prospective study in a mixed intensive care unit. Surgery 110:303, 1991.
23. Stoutenbeek ChP, van Saene HKF, Miranda DR, et al: The effect of selective decontamination of the digestive tract on colonization and infection rate in multiple trauma patients. Intens Care Med 10:185, 1984.
24. Ledingham IMcA, Alcock SR, Eastaway AT, et al: Triple regimen of selective decontamination of the digestive tract, systemic cefotaxime, and microbiological surveillance for prevention of acquired infection in intensive care. Lancet 1:785, 1988.
25. Gastinne H, Wolff M, Delatour F, et al: A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. N Engl J Med 326:594, 1992.
26. Knaus WA, Draper EA, Wagner DP, et al: An evaluation of outcome from intensive care in major medical centers. Ann Intern Med 104:410, 1986.
27. Driks MR, Craven DE, Celli BR, et al: Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. N Engl J Med 317:1376, 1987.
28. Craven DE, Driks MR: Nosocomial pneumonia in the intubated patient. Semin Respir Infect 2:20, 1987.
29.
Kingston GW, Phang PT, Leathley MS: Increased incidence of
nosocomial pneumonia in mechanically ventilated patients with subclinical
aspiration. Am J Surg 161:585, 1991.
Table 1. Relationship Between the Patient Groups
and
Pneumonia
Patient
Pneumonia
Groups Patients (n) Cases (n,%)
Major
surgical intervention 47 11(23.40)
Trauma 14 5(35.71)
Medical
treatment 6
5(35.71)
Table
2. Results of the Nasogastric
Tube Aspirate and Sputum Cultures
Culture
Results No./Percent
NG tube aspirate culture (–) and sputum culture (–) 85 (69.67%)
NG tube aspirate culture (+) and sputum culture (–) 7 (5.74%)
NG tube aspirate culture (–) and sputum culture (+) 6 (4.92%)
NG tube aspirate
culture (+) and sputum culture (+)
20 (16.40%)
(isolated microorganisms
were the same)
NG tube aspirate
culture (+) and sputum culture (+)
4 (3.27%)
(isolated microorganisms
were different)
Total 122 (100%)
NG,
nasogastric.
Table 3. Microorganisms Isolated From the Nasogastric
Tube Aspirate Samples
Microorganism No.
Coagulase (+) Staphylococcus 10
Klebsiella 8
Pseudomonas 4
E. coli 3
Coagulase (–) Staphylococcus 2
Alpha hemolytic Streptococcus 1
Pseudomonas/coagulase (+) Staphylococcus 1
Pseudomonas/Klebsiella 1
Coagulase (+) Staphylococcus/Candida 1
Total
31
Table 4. Microorganisms Isolated From the Sputum
Samples
Microorganism No.
Coagulase (+) Staphylococcus 11
Pseudomonas 8
Klebsiella 5
Coagulase (–) Staphylococcus 2
Pneumococcus 1
Branhamella 1
Coagulase (+) Staphylococcus/E. coli 1
Pseudomonas/coagulase (+) Staphylococcus 1
Total 30
Table 5. Relationship Between the Gastric Colonization
and Pneumonia
Pneumonia (+) Pneumonia (–) Total
Gastric colonization (+) 14* 3 17
Gastric colonization (–) 3* 47 50
Total 17 50 67
The pneumonia incidence was
significantly higher in the group having pathologic gastric colonization
than in the group not having pathologic gastric colonization.
*P <0.0001 by the chi square statistics with Fisher exact test.
Figure
1. Relationship between the APACHE II scores and pneumonia.
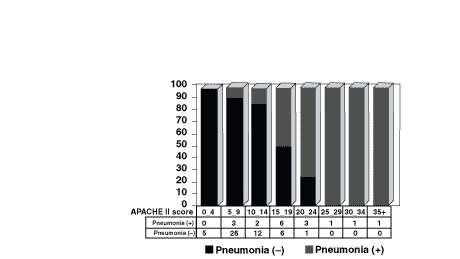
Figure 2. Relationship between the use of ranitidine
and gastric colonization.
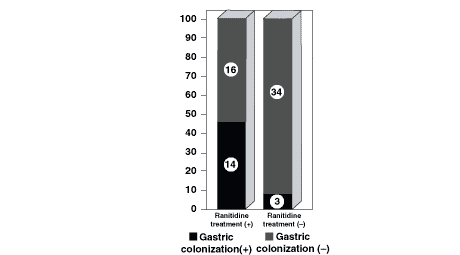
Figure 3. Correlation between the results of nasogastric
tube aspirate cultures and sputum cultures.
©2000-2013. All Rights Reserved. Veterinary Solutions LLC
2Checkout.com is an authorized retailer for The
Journal of Applied Research