|
The JOURNAL of APPLIED RESEARCH In Clinical and Experimental Therapeutics |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The Journal of Applied Research |
Biocompatibility
and Biodegradation of High Doses
of FloSeal® in Rats
O. Reuthebuch, MD*
U. Moehrlen, MD*
M.O. Kurrer, MD†
U. Stammberger, MD*
M.L. Lachat, MD*
M. Turina, MD*
*Clinic for Cardiovascular Surgery
†Institute of Clinical Pathology, Department of Pathology,
University Hospital Zürich
Rämistrasse 100, CH-8091 Zürich, Switzerland
KEY WORDS: tissue adhesives, thrombin, gelatin, hemostasis, biocompatible materials
ABSTRACT
Background: FloSeal® is a new topical hemostatic agent that has proven its efficacy in cardiac and vascular surgery. The basic components of FloSeal® are thrombin and gelatin, both of which have been shown to be safe for more than 3 decades in clinical use. Although biological glues are frequently used in pediatric cardiac surgery, deliberate use is often required to obtain reliable hemostasis. The aim of the present study was to use a rat model to evaluate adverse effects, if any, of high doses of FloSeal®.
Method: FloSeal®, 3 mL (equivalent to 900 mg/kg body weight), was injected under anesthesia into the peritoneal cavity of 12 male Sprague-Dawley rats, and the animals were allowed to recover from sedation. Following the procedure, the animals were observed daily and weighed twice a week. Six animals (group 1) were killed 30 days after treatment, and six animals (group 2) were killed after 60 days. Laparotomy was performed on all rats, and adhesions were evaluated macroscopically and microscopically. In addition, any remaining amounts of FloSeal® were carefully assessed.
Results: All animals survived the treatment in good condition, and none appeared to be in pain or distress during the study period. After the animals were killed, no adhesions or gross lesions were found macroscopically. Histological evaluation revealed slight alterations (subperitoneal fibrosis, vasculogenesis) in two animals in group 1 and in one animal in group 2 (subperitoneal fibrosis). No remnants of FloSeal® were found in either group.
Conclusion: High doses of FloSeal® were biocompatible and biodegraded in this rat model. FloSeal® was resorbed within 30 days, and microscopic examination revealed no relevant histopathologic effects. High doses of FloSeal® appear to be safe and well tolerated.
INTRODUCTION
Biological glues such as fibrin glue or hemostatic agents are frequently used in cardiac or vascular surgeries to control diffuse bleeding.1–3 However, these agents are also being used increasingly to control more aggressive bleeding that cannot be stopped using standard surgical approaches due to inaccessibility, tissue fragility, or small size (pediatric) of the surgical area.
Aside from systemic measures (such as normalization of coagulation factors through transfusions, infusion of aprotinin, auto-transfusion or cell-saving), the application of topical hemostatic agents such as gelatin-sponges, cellulose, fibrin-glue, and, more recently, FloSeal® (FloSeal Matrix Hemostatic Sealant; Fusion Medical Technologies, Fremont, CA) are helpful means of addressing troublesome intraoperative bleeding.4,5 FloSeal® is a combination of cross-linked gelatin matrix and thrombin. The gelatin particles in FloSeal® have the potential to swell on contact with fluids, resulting in increasing adherence to wet, irregular, oblique, or vertical surfaces, and if mechanical pressure is applied. In addition to the effects of thrombin, which converts fibrinogen into fibrin monomers that polymerize to form a fibrin clot, the gelatin matrix acts synergistically, resulting in sealing of the wound. The established matrix then facilitates wound healing. We have recently shown that the average amount of FloSeal® adult patients required was about one syringe (about 5 g).6 However, larger amounts of FloSeal® may be required in some situations.
The aim of this study was to determine the safety of FloSeal® when used at fairly high dosages, and to evaluate the macroscopic and histological response of biological tissue at these high exposures. Additional objectives were to quantify the potential development of adhesions and evaluate the biodegradation time of high doses of this novel hemostatic sealant. Therefore, a tenfold greater than average dose of FloSeal®, based on body weight, was injected into the peritoneal cavity of rats, which are the generally accepted standardized models for toxicology studies.
MATERIAL AND METHODS
The Ethical Committee of the University of Zurich, Switzerland, approved the experimental protocol for this study prior to initiation. All animals were handled in accordance with the guiding principles on care and use of animals approved by the American Physiological Society, and the investigations conformed to the Guide for Care and Use of Laboratory Animals published by the US National Institute of Health. Historical data on the normal growth curves for this strain of rat were used as a negative control. Any animal that appeared to be in pain or distress was to be reported to the study director for appropriate evaluation and treatment, and any animals deemed untreatable would be euthanized.
Each syringe of FloSeal® contains 4 grams of material (equivalent to 4 mL). If a 70 kg human were treated with 15 syringes of FloSeal®, this patient would receive a total of 857 mg/kg/body weight of FloSeal®. For practical reasons, the dose was set to 900 mg/kg/body weight, equivalent to 15.75 syringes. Therefore, a rat weighing 350 g was injected with 313 mg (0.31 mL) FloSeal®. Individual dosage was adapted to the actual weight of each rat.
Fusion matrix was prepared per its instructions for use. The 5000 IU of lyophilized bovine thrombin were reconstituted in 0.8 mL of sterile saline using a 1 mL syringe. Using the dispersion needle and syringe assembly supplied in the FloSeal® kit, 0.5 mL of the thrombin solution was injected into the gelatin matrix syringe under slight and consistent pressure. The FloSeal® could be used for up to 2 hours after mixing.
Experimental Protocol
Twelve male Sprague-Dawley rats with a mean weight of 368.8 ± 10.7 g were included in this study. The number of rats selected allows statistically significant data to be generated. All animals were anaesthetized prior to surgery. A sponge soaked with the anesthetic Metofane® was placed under a wire grid on the bottom of a basin serving as an anesthesia chamber. The rats were placed on a raised wire mesh, and a lid was placed on the basin. Skin contact with inhalation anesthetic had to be avoided to prevent potential overdosing from cutaneous absorption. With consistent control of breathing, this kind of anesthesia allows several minutes for surgery. Anesthetized rats were weighed and the weights recorded.
The rats were placed in a supine position and the calculated amount of FloSeal® injected intraperitoneally via a 14 guage needle, preferably into the lower left or right quadrant to prevent organ injury. After the needle was withdrawn the site of puncture was carefully massaged to prevent leakage and to disperse the FloSeal® in the abdominal cavity. The rats were randomly assigned to two experimental groups, each containing 6 rats (group 1 to be euthanized at day 30 after treatment, and group 2 to be euthanized at day 60 after treatment). All rats were allowed to recover from sedation and returned to their home cages. All rats were observed daily and weighed twice a week.
At the appropriate termination time, the animals were killed by placing them in a closed chamber saturated with 80% CO2 for 15 minutes. The rats underwent necropsy, and any lesions were carefully assessed. Following a laparotomy, the abdominal wall was spread open and examined macroscopically. Appropriate photographs were taken.
The presence or absence of adhesions in the abdominal cavity was estimated and quantified on a scale of 0 to 5 (0 = no adhesions; 5 = severe adhesions). The presence of any FloSeal® was also quantified on a scale of 0 to 5 (0 = no material left, 5 = a lot of material left). Afterward, biopsies were taken of the abdominal wall in each quadrant and stored in 10% buffered neutral formalin solution. Tissue samples were evaluated histologically to assess inflammatory reactions, foreign bodies, and reactions due to foreign bodies.
RESULTS
All animals survived the procedure, and recovery was uneventful. No animal had to be killed earlier than the established date. Weight gain over the 30 and 60 day postoperative periods was within the normal range for this strain of rats, and none of the animals lost weight during the observation period. The mean body weight after 30 days was 457.5 ± 17.5 grams (n=12), and the mean weight of the rats after 60 days was 515.0 ± 26.7 grams (n=6).
At necropsy, gross and histologic examination showed no abnormal or unexpected responses to the applied FloSeal®. FloSeal® was not found in any areas other than in the left lower quadrant. The degree of adhesion-formation and amount of residual FloSeal® observed macroscopically are shown in Table 1.
A small amount of FloSeal was observed in only one of six rats at day 30. FloSeal® was completely absent in all the 60-day animals. Adhesions were observed in only one of six animals in group 1 (30-day). These were scored as Grade 2 (according to the previously mentioned scale). This was the only animal that also showed a presence of FloSeal® remnants. No adhesions were observed in any animals in group 2 (60-day).
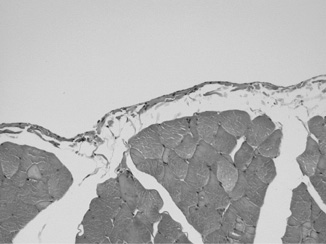
Representative photomicrographs of the histologic tissue sample evaluation are presented in Figure 1. Histologic examination revealed normal findings, showing normal serosa and underlying muscle with only minimal signs of fibrosis (Figure 1).
DISCUSSION
Bleeding in cardiac surgery, particularly after cardiopulmonary bypass with its change in hemostatic cascades, is a common problem, occurring in up to14% of cases.7 With the increase in minimally invasive, robotically enhanced, or beating heart operations, as well as with more complex situations in pediatric cardiac surgery, the frequency and intensity of bleeding complications may raise in the future. This will lead to the need for reliable hemostatic agents in addition to the classic suture.
Prior investigations at this institute demonstrated the efficacy of a new sealant (FloSeal Matrix) in stopping bleeding ranging from oozing to spurting in peripheral vascular surgery.6 A recently published paper by Oz et al8 reported comparable data in cardiac surgery. FloSeal®, a mixture of gelatin and thrombin, swells up to 20% by volume when in contact with bodily fluids, resulting in a slight pressure on the wound. Therefore, unlike other synthetic glues, it is not washed away, even on a bloody surface under heparinization, and leads to hemostatic success in a reasonable time.8,9
With the increasing experience with FloSeal® in adult cardiac surgery, it is now also gaining popularity in pediatric cardiac surgeries. Due to the complexity of pediatric surgical procedures aggravated by small body size, the need to address oozing or even spurting is frequent. However, based on body weight, pediatric patients may require a much higher dosage of FloSeal® than is used in adult cardiac surgery. Thus, the aim of this study was to evaluate the safety of high doses of FloSeal® in an animal model, prior to its use in humans. The rat model was chosen because of its wide acceptance as a standardized model for toxicology studies.
Clinically, fibrin-based sealants composed of fibrinogen and thrombin, and “French glue” composed of gelatin, resorcinol, formaldehyde, and glutaraldehyde (GRFG) are widely used as sealants and for hemostasis.10 Although the fibrin sealants are mainly absorbed in about 30 days, GRFG may be present for more than 6 months.9 The results of the present study show that FloSeal®, even at a dosage tenfold higher than usual, undergoes complete biodegradation within 30 days and is essentially absent at after 60 days. Furthermore the lack of any abnormal reactions in these animals suggested the absence of pain and discomfort with these high doses of FloSeal®. Gross and histological examinations showed no abnormal or unexpected interactions between FloSeal and body tissues, since adhesion formation was not stimulated. In contrast, the use of GFRG glue results in inflammatory tissue reactions, leading to severe adhesion formations.11 Although adhesion formation after administration of fibrin sealants is controversial, most publications report enhanced adhesion formation.13,14 Surgical procedures to correct congenital cardiac abnormalities in children are often palliative, and additional surgical steps are required until definitive correction is achieved. In this context, the absence of adhesions following the use of high doses of FloSeal® seen in this study could be beneficial. Additional studies are needed to categorically establish that FloSeal® has anti-adhesion properties.
CONCLUSIONS
The data from this animal experiment show that high doses of FloSeal® are safe and fully biocompatible, and that FloSeal® undergoes a rapid and complete biodegradation within 30 days. With the reported results in mind, we plan to begin clinical evaluation of FloSeal®, focusing on pediatric cardiac surgery.
ACKNOWLEDGEMENTS
The authors are grateful to Narinder S. Shargill, Ph.D. (Dublin, CA) for help with the preparation of this manuscript.
REFERENCES
1. Moak E: Hemostatic agents: Adjuncts to control bleeding. Today`s OR Nurse 13:6–10, 1991.
2. Zwischenberger JB, Brunston RL Jr, Swann JR, Conti VR: Comparison of two topical collagen-based hemostatic sponges during cardiothoracic procedures. J Invest Surg 12:101–106, 1999.
3. Cooper QW, Falb RD: Surgical adhesives. Ann NY Acad Sci 146:214-224, 1968.
4. Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood 76:1680–1697, 1990.
5. Goldstein DJ, DeRosa CM, Mongero LB: Safety and efficacy of aprotinin under conditions of deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg 110:1615–1621, 1995.
6. Reuthebuch O, Lachat ML, Vogt P, Schurr U, Turina M: FloSeal®: A new hemostyptic agent in peripheral vascular surgery. Vasa 29(3):204–206, 2000.
7. Czer LSC: Mediastinal bleeding after cardiac surgery: Etiologies, diagnostic considerations and blood conservation methods. J Cardiothorac Anesth 3:760–765, 1989.
8. Oz MC, Cosgrove DM III, Badduke BR: Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg 69:1376–1382, 2000.
9. Basu S, Marini CP, Baumann FG, et al: Comparative study of biological glues: Cryoprecipitate glue, two-component fibrin sealant, and “French” glue. Ann Thorac Surg 60:1255–1262, 1995.
10. Bachet J, Goudot B, Dreyfus GD, et al: Surgery for acute type A aortic dissection: The Hopital Foch experience (1977–1998). Ann Thorac Surg 67:2006–2009, 1999.
11. Ennker J, Ennker IC, Schoon D, et al: The impact of gelatin-resorcinol glue on aortic tissue: A histomorphological evaluation. J Vasc Surg 20:34–43, 1994.
12. Walker JD, Kratz JM, Basler CG, et al: Fate of gelatin-resorcinol-formaldehyde/glutaraldehyde adhesive on femoral vessel morphology. J Surg Res 71:73–78, 1997.
13. Houston KA, Rotstein OD: Fibrin sealant in high-risk colonic anastomoses. Arch Surg 123(2):230–234, 1988.
14. Gauwerky JF, Mann J, Bastert G: The effect of
fibrin glue and peritoneal grafts in the prevention of intraperitoneal
adhesions. Arch
Gynecol Obstet 247(4):161–166, 1990.
Table 1. Degree
of Adhesion Formation and Amount of Residual FloSeal®
Group 1 Group 2
(30-day) (60-day)
Animal No. 1 2 3 4 5 6 7 8 9 10 11 12
Adhesion Score* 0 0 2 0 0 0 0 0 0 0 0 0
Residual FloSeal® Score† 0 0 1 0 0 0 0 0 0 0 0 0
*Adhesion Score: 0 = no adhesions, 5 = severe adhesions.
†Residual FloSeal® Score: 0 =
no material left, 5 = lot of material left
Figure 1. Histological
section of the peritoneum (Hematoxylin & Eosin staining, original
magnification X20). A thin serosal membrane with scant connective
tissue and unremarkable underlying abdominal wall muscle is seen.
Inflammatory infiltrates within the serosal or subserosal tissues
are absent. There is no fibrinous exudate on the peritoneal surface,
nor is there a proliferation of granulation tissue. Fibrous tissue
within the serosal membrane is at most focally minimally increased.
©2000-2013. All Rights Reserved. Veterinary Solutions LLC
2Checkout.com is an authorized retailer for The
Journal of Applied Research