|
The JOURNAL of APPLIED RESEARCH In Clinical and Experimental Therapeutics |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The Journal of Applied Research |
Improved
Healing of Split
Thickness Skin Graft Donor Sites
Bishara S. Atiyeh, MD, FACS*
Christian A. Al-Amm, MD†
Ali A. Nasser, MD†
*Associate
Clinical Professor of Surgery
†Chief
Resident
Division
of Plastic & Reconstructive Surgery
American
University of Beirut Medical Center
Beirut,
Lebanon
KEY WORDS: skin grafts, wound healing, epidermal dressing
ABSTRACT
Cosmetically unacceptable pigmentation changes and hypertrophic scarring often complicate split thickness skin graft (STSG) donor sites. In fact, patients often ask about other alternatives when confronted with the need to harvest STSG, particularly because they fear additional disfigurement. Because recent evidence suggests that a moist environment promotes more optimal healing, we conducted a limited controlled and comparative study to assess the effect of a newly introduced burn ointment MEBO (Moist Exposed Burn Ointment) (Julphar Gulf Pharmaceutical Industries, Ras Al-Khaimah, UAE) on re-epithelialization and healing of STSG donor sites as compared with the conventional Sofra-Tulle semi-open dressing (Roussel Laboratories Ltd., Uxbridge, England). We observed that MEBO treated areas were completely reepithelialized within 5 to 6 days while conventionally treated areas required 10 to 12 days to heal.
Conventionally treated areas were also less hyperemic and less pigmented. Photographs from both treatment groups were retrospectively evaluated along a defined assessment scale; these also revealed significantly better cosmetic results for ointment treated areas. The most striking difference in our opinion, however, was the lack of epidermal sliding in the MEBO treated areas. This result is similar to normal skin and suggests a profound structural difference for MEBO-induced healing. The epidermis of the basal layer in the MEBO treated areas seemed to be better anchored to the underlying dermis, theoretically making MEBO treated areas less vulnerable to frictional forces. These study results imply that the application of MEBO will result in equally good re-epithelialization for similar partial thickness cutaneous wounds caused by dermabraision, chemical peeling, or laser treatment for skin resurfacing.
INTRODUCTION
Split thickness skin grafting (STSG) is a frequently used technique for covering soft tissue and skin defects. Its wide range of applications makes it valuable not only to plastic and reconstructive surgeons but also to other surgical specialties.1 The technique evolved from use in the back alleys of India in pre-Christian times to become one of the most valuable clinical tools in modern surgery.2 Techniques for caring for the skin graft site to assure an adequate graft and prompt wound healing are well established and are widely accepted and applied by workers in the field.
Unfortunately, however, similar consensus does not exist regarding optimal donor site care or what dressing would result in the best healing and most cosmetically acceptable result.1,3 STSG donor site areas have been traditionally dressed with low-adherent wound contact paraffin gauze or antibiotic-impregnated tulle gras and covered by a secondary dressing made of gauze and absorbent padding.2,4–6 These dressings are relatively inexpensive and do not require specific expertise for application or post-operative management. However, during the peri-operative period, patients complain more often of discomfort or pain at the donor area than at the graft site itself.3 Additionally, the poor cosmetic appearance of donor sites after healing is not readily accepted.
In theory, an ideal STSG donor site dressing should be easy to apply, promote rapid re-epithelialization, and be pain free, infection free, and relatively inexpensive.3 It must also be immunologically tolerated and, above all, result in good quality healed skin with minimal scarring.4 Feldman1,3 categorized STSG donor site dressings into five main categories: open, semi-open, occlusive, semi-occlusive, and biological. The open method, in which no dressing is applied, tends to be painful and is associated with prolonged healing time.1,6–8 It is definitely the cheapest of possible dressings, provided the prolonged healing time does not result in extended hospitalization.1
Most authors, however, prefer to protect the donor site wound from trauma and infection6 by covering it with a low-adherent, Vaseline-impregnated fine mesh gauze. Medicated layers such as Scarlet Red, Xeroform, Adaptic, Jelonet, and Sofra Tulle are often used as well.1,6 The wound contact layer is then covered by a secondary, semi-open dressing consisting of gauze and absorbent padding.4 Such dressings are usually bulky and restrict mobility, and they do not alleviate pain at the donor site.4 Their fine mesh also allows the egress of fluid and bacteria.3 As the dressing dries, fibrin from the wound bed causes temporary bonding of the dressing to the wound,9 and re-epithelialization procceds beneath it.1
Biobrane is a semi-open dressing; however, it has a different mechanism of attachment: Its collagen peptide content enables it to be incorporated into the wound. Unfortunately, although it is more comfortable than mesh gauze, Biobrane has been associated with a high rate of infection.1,3,10
Early occlusive dressings consisted of fine mesh gauze covered by an impermeable membrane. These were quickly abandoned, however, because of potential bacterial proliferation and because they were difficult to apply to areas other than the extremities.3,11 Duoderm is a new type of occlusive dressing based on the principle of moist wound healing.1,3,4 It provides a moist, non-adherent environment over the graft donor site, permitting rapid, infection-free reepithelialization.12–15 Although Duoderm dressing appears to be ideal, when used on large surface areas, it causes an unmanageable amount of dressing exudate, and becomes cost ineffective and labor intensive.1 It has also been associated with the formation of granulomas and with increased inflammation and possibly increased scarring.16
Semi-occlusive dressings comprise the group of clear films often referred to as “SAM” (synthetic adhesive moisture-vapor-permeable) dressings. They are also bacteria and liquid impermeable.1,3,12 However, as with Duoderm, fluids tend to collect beneath these dressings, requiring frequent drainage or replacement.3,6
Biological dressings include the re-application of excess autogenous skin graft, human cadaver skin, porcine skin, amniotic membrane, and cultured keratinocyte grafts.1,6 However, although autografting of excess skin taken from the same donor site is the ideal biological dressing, all other biological dressings have great disadvantages. The most notorious of these are their antigenicity and their potential for transmitting viral and bacterial pathogens. Their higher cost can also preclude their routine use, though in extreme conditions, such as extensive burns, they can be life saving.
MATERIAL AND METHODS
MEBO (Moist Exposed Burn Ointment; Julphar Gulf Pharmaceutical Industries, Ras Al-Khaimah, UAE) is a topical agent primarily used to treat burn injuries. Because the basic pathology of second degree burns closely mimics many aspects of STSG donor sites, the effect of MEBO on STSG donor site healing was investigated in a limited clinical trial involving 15 consecutive patients. Preliminary study results were already reported.17,18
To avoid patient to patient variation, each patient acted as both test subject and control. Moreover, to minimize the fact that different parts of the body with different skin thickness have differenct re-epithelialization and healing potentials, the same donor site was used as both study and control site. Therefore, any observed differences could be attributed to the treatment itself rather than to other variables. All patients were Caucasians, with Fitzpatrick skin types II and III, with an age range of 5 to 65 years. A Padget Electric Dermatome was used to harvest a 0.12 inch thick skin graft from the thigh in all patients. The recommended MEBO application schedule–every 6 hours in a thin layer following scraping the previously applied layer–was not practical in our hospital setting. Therefore, we applied a thick layer of MEBO on half of the STSG donor site surface area at the time of surgery. That layer was then covered by a thin non-occlusive semi-open dressing. The ointment was then reapplied and the dressing changed daily until full re-epithelialization was observed. Standard conventional dressing consisting of an antibiotic impregnated Vaseline gauze, Sofra Tulle (Roussel Laboratories Ltd, Uxbridge, UK) was applied to the other half of the donor site intra-operatively and covered by a bulky gauze dressing held in position by an elastic bandage. The bandage was removed 24 to 48 hours later and the now adherent gauze was kept uncovered and undisturbed until spontaneous separation occurred.
Parameters evaluated included speed of re-epithelialization, analgesia, and the cosmetic appearance of the healed donor site. Because the degree of hyperemia, pigmentation, and cosmetic appearance are subjective measures and difficult to quantify, photographs of the adjacent study and control areas were taken at regular intervals (Figure 1). We believe this to be superior to any kind of artificial scale19 that would result in assigning numerical values to different color shades or cosmetic results. Although we did not use a pain evaluation scale such as the Pain Analogue Thermometer described by Choiniere et al,20 patients could comment easily on pain and comfort since they could directly and immediately compare the two types of dressings. Patients or their parents (for pediatric patients) were also asked about their preferences and their degree of satisfaction regarding results. Photographs of the healed donor sites were also evaluated retrospectively by an observer using the image assessment guidelines described by Beausang et al.19 The longest follow up in this study was 1.5 years.
RESULTS
Although statistical analysis was not performed because of the small number of patients in each group, we can report that MEBO treated areas were completely re-epithelialized within 5 to 6 days, whereas the conventionally treated areas required 10 to 12 days to heal. The MEBO treated areas were also markedly less hyperemic and less pigmented. Final cosmetic appearance and patient satisfaction were also higher for MEBO treated areas. Finally, the retrospective image assessment scores were also better for the MEBO treated areas (Figure 1). Differences in pain assessment, however, were less marked. This is probably because MEBO was not applied every 6 hours as is recommended to achieve optimal analgesia. Nevertheless, all patients were noted to be more comfortable with MEBO, probably because of its rapid healing time and therefore shorter painful period. In one adult male patient, hair regrowth proceeded at a faster rate in the MEBO treated area than in the control area. Finally, all patients showed a lack of epidermal sliding in the MEBO treated area that was comparable to normal skin. Variable degrees of epidermal sliding was seen in control areas.
DISCUSSION
The overall efficacy of various dressings is usually determined based on time to healing, associated pain, infection rates, and expense.5 With few exceptions,4 researchers did not consider the quality of healed skin, the cosmetic appearance after healing, or patient satisfaction and preferences. Researchers have always stressed the preference of obtaining STSG grafts from less visible donor surface areas because of the likelihood of cosmetically unacceptable scarring. Nevertheless, they did not seem to appreciate the need to develop dressings that would promote optimal re-epithelialization and wound healing while reducing hypertrophic scarring and pigmentation changes. We believe that the ultimate cosmetic appearance of STSG donor sites is important to most patients and should take precedence over other considerations because a donor area scar often represents an added distress to an already stressful situation.
According to the Wound Healing Society, a wound is a disruption of normal anatomic structure and function. Wound healing is a complex series of events characterized by inflammation, which does not culminate in tissue regeneration but rather in tissue restoration.21 Healing involves three processes: epithelialization, connective tissue deposition, and contraction. The contribution of each process varies according to the type of wound.22 Epithelialization of partial thickness wounds results in wound resurfacing and restoration of the stratified squamous epithelium that protects the body from fluid loss, bacterial invasion, electromagnetic radiation, and general trauma.23 Connective tissue deposition replaces the underlying damaged dermis.
More recent evidence in the literature suggests that good hydration is the single most important external factor responsible for optimal wound healing.24–28 Since Winter29 proposed his classic hypothesis that the optimum environment for epithelialization is moist, few have challenged him. However, some researchers proposed that the optimum environment would be an intermediate gelatinous environment between moist and dry, such as under highly vapor-permeable dressings.30,31 Still other experts demonstrated accelerated healing of full-thickness skin wounds in a wet rather than moist environment.24 Irrespective of this apparent controversy, experts agree that allowing traumatized or ischemic tissue to dehydrate produces further tissue loss by transforming the “zone of stasis” adjacent to the injured area into a “zone of necrosis.”24 In the natural course of events, dermis or deeper subcutaneous tissues devoid of epidermal protection lose water to the atmosphere. The uppermost layers become dehydrated and incorporated in the scab trapping, some of the leukocytes that crowd to the wound surface.32
Despite this mounting evidence and appreciation of the biologic factors of moist environments and their ability to promote rapid infection-free re-epithelialization with less pain,1,3,4,6,15,33,34 the advantages of water-impermeable occlusive dressings on wound healing are often offset by their impracticality, particularly when applied to large split thickness donor site areas.1 MEBO, which is the basis of MEBT (moist exposed burn therapy) popularized two decades ago by Xu35 from the Beijing Burn Center offers the advantages of a moist environment for wound healing in addition to the advantage of avoiding cumbersome, bulky, and expensive dressings. MEBO has been patented in the United States since 1995. Its active component is ß-sitosterol in a base of beeswax, sesame oil, and other components. Clinical and experimental studies reported in the Chinese literature have demonstrated that MEBO markedly reduces evaporation from the wound surface.36 MEBO has an inhibitory effect on smooth muscle cells that is dose related37 and has no evident effect on the humoral and cellular immune defense mechanisms.38
Although MEBO does not demonstrate in vitro bacteriostatic or bactericidal activity, probably because its oily composition does not allow proper diffusion in a watery culture medium,39,40 research shows it to have similar in vivo action to sulfadiazene silver in controlling burn wound sepsis and systemic infection with Pseudomonas aeroginosa.41 Research demonstrated that MEBO exhibited a statistically significant wound healing potential on rabbit corneal epithelium as compared with saline, homologous serum, vitamin A, and dexamethasone.42 Moreover, rabbit skin burns healed at a much faster rate with better quality scars when treated with MEBO than similar burns treated with Vaseline, with demonstrable histologic differences on repeated serial biopsies.43 A recent report found MEBO to be a useful alternative for treating partial thickness facial burns because of its convenient method of application, which allows easier assessment of healing progression.44
Although patient satisfaction and preferences are highly subjective, the reaction from patients and their families left little room for doubt and could not be qualified as biased. The evidence provided by serial photographs of early and late postoperative cosmetic results illustrate a superiority for MEBO as well. Ointment treated areas were clearly less hyperemic immediately after re-epithelialization. Hyperemia cleared almost completely from the MEBO treated areas within 3 months, while it persisted for more than 6 months in the control areas. This cannot be explained by increased vasoconstriction because the decreased hyperemia persisted after MEBO application was stopped. Additionally, MEBO has an inhibitory effect on the smooth muscle cells that lead to vasodilatation and hyperemia. Therefore MEBO application improves local microcirculation and, as demonstrated in burn injury, increases blood flow to the zone of stasis.38,40
The lessened hyperemia with MEBO also cannot be explained by reduced neoangiogenesis in the healing area. Histologic examination of biopsies taken from experimental rabbit burns treated with MEBO clearly demonstrated increased capillary density with larger lumen as compared with similar biopsies taken from rabbit burns treated with Vaseline.43 The decreased hyperemia most likely is due to increased opacity of tissues overlying the subdermal layer, caused by thicker regenerating epithelium or dermis or both. This observation, however, needs validation by proper histologic studies. Unfortunately these studies cannot be performed with human subjects. Decreased hyperemia could also be explained by the fact that increased tissue moisture decreases capillary activity and reduces hyperemia and collagen deposition.45,46
Observed pigmentation differences between the two groups could also be explained by the fact that prolonged healing time observed with the conventional dressing may predispose the healing area to a more pronounced inflammatory reaction, resulting in post-inflammatory hyperpigmentation. Although frank infection was not observed in either group, the areas treated with Sofra Tulle suffered frequently from patchy forceful early separation of the adherent gauze with bleeding and subsequent limited suppurative infection, which may also have exacerbated the inflammatory response. In one patient, forceful separation was marked and repetitive; the traumatized bed became severely hyperpigmented and slightly hypertrophic. In fact, some of the MEBO treated areas became hypopigmented, which may suggest an inhibitory action on melanocytic action in the re-epithelialized areas.
Epidermal sliding is a pathologic finding described originally in conjunction with chemical peeling in order to clinically determine the depth of the peel.17,47,48 Epidermal sliding, when present, indicates separation of the epidermal layers from the underlying dermis, allowing the denatured epidermis to slide over the elastic dermis when pinched. Epidermal sliding disappears when chemical denaturation of tissues proceeds deeper in the dermis, binding epidermis and dermis. Strikingly, epidermal sliding was found in most areas treated with the conventional dressing for up to few months after healing; it was totally absent from the MEBO treated areas as well as from normal adjacent skin. This confirms that the difference in re-epithelialization between the two groups is not only one of speed and time but rather a profound structural difference that warrants further investigation. The epidermis of the basal layer in the MEBO treated group seemed to be better anchored to the underlying dermis, which makes MEBO treated areas theoretically less vulnerable to frictional forces.17,18
CONCLUSIONs
Although the full action of MEBO has not yet been fully explored, its ease of application, documented safety, reasonable cost, and evident capacity to promote speedy healing with excellent cosmetic outcome make it close to be the ideal dressing for STSG donor sites. Patient satisfaction was very high in general, and MEBO was unequivocally preferred over antibiotic-impregnated, low-adherence wound contact Vaseline gauze dressing. MEBO application may also be indicated for similar partial thickness cutaneous wounds such as those seen after dermabrasion, chemical peeling, or laser treatment for skin resurfacing. Good re-epithelialization following laser burns has already been documented in an animal model.49 Preliminary results of this study were already reported.17,18 Obviously, more controlled clinical trials are still needed.
REFERENCES
1. Feldman L: Which dressing for split-thickness skin graft donor sites? Ann Plast Surg 27:288– 291, 1991.
2. Davis JS: story of plastic surgery. Ann Surg 113:651–656, 1941.
3. Feldman D, Rogers A, Karpinski R: A prospective trial comparing Biobrane, Duoderm, and Xeroform for skin graft donor sites. Surg Gynecol Obstet 173:1–5, 1991.
4. Vanstraelen P: Comparison of calcium sodium alginate (KALTOSTAT) and porcine xenograft (E-Z DERM) in the healing of split-thickness skin graft donor sites. Burns 18:145–148, 1992.
5. Morris WT, Lamb A: Painless split skin donor sites: A controlled double-blind trial of Opsite, Scarlet Red and Bupivacaine. Austral N Z J Surg 60:617–620, 1990.
6. Kelton P Jr: Skin grafts. Select Readings Plast Surg 7(2):1–25, 1992.
7. Brady SC, Snelling CFT, Chow G: Comparison of donor site dressings. Ann Plast Surg 238–243, 1980.
8. Salisbury RE, Wilmore DW, Silverstein P, Pruitt BA: Biologic dressings for skin graft donor sites. Arch Surg 106:705–706, 1976.
9. Zapata-Sirvent R, Hansbrough JF, Carrol W, et al: Comparison of Biobrane and Scarlet Red dressings for treatment of donor site wounds. Arch Surg 120:743–745, 1985.
10. Griswold JA, Grube BJ, Engrav LH, et al: Determinants of donor site infections in small burn grafts. J Burn Care Rehabil 10:531–535, 1989.
11. Friedman GD, Capozzi A, Pennisi VR: Care of the split thickness skin graft donor site. J Trauma 14:163–167, 1984.
12. Alvarez OM, Mertz PM, Eaglestein WH: The effect of occlusive dressings on collagen synthesis and reepithelialization in superficial wounds. J Surg Res 35:142–148, 1983.
13. Varghese MC, Balin AK, Carter M, Caldwell D: Local environment of chronic wounds under synthetic dressings. Arch Dermatol 122:52–57, 1986.
14. Leicht P, Siim E, Sorensen B: Treatment of donor sites—-Duoderm or Omiderm. Burns Incl Therm Inj 15:7–10, 1989.
15. Nemeth AJ: Faster healing and less pain in skin biopsy sites treated with an occlusive dressing. Arch Dermatol 127:1679–1683, 1989.
16. Reuterving CO, Agren MS, Soderberg TA, et al: The effects of occlusive dressings on inflammation and granulation tissue formation in excised wounds in rats. Scand J Plast Reconstr Surg 23:89–96, 1989.
17. Atiyeh BS, Ghanimeh G, Kaddoura IL, Ioannovich J: Split-thickness skin graft donor site dressing: Preliminary results of a controlled, clinical comparative study of MEBO and Sofra-Tulle. Ann Plast Surg 46:87–88, 2001.
18. Atiyeh BS, Ioannovich J, Al-Amm CA: Pansements De Sites Donneurs De Greffe De Peau Mince: Resultats Preliminaires D’une Etude Clinique Limitee Comparative De “MEBO” Et De “Sofra-Tulle.” Brulures, Revue Française de brûlologie 1:155–161, 2000.
19. Beausang E, Floyd H, Dunn KW, et al: A new quantitative scale for clinical scar assessment. Plast Reconstr Surg 102:1954–1961, 1998.
20. Choiniere M, Auger FA, Latarjet J: Visual analogue thermometer: A valid and useful instrument for measuring pain in burned patients. Burns 20:41, 1994.
21. Cohen KI: The biology of wound healing. Contemp Surg Suppl Sept:4–8, 2000.
22. Grinnel F: Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 124:401–404, 1994.
23. Cohen KI, Diegelmann RF, Yager DR, et al: Wound care and wound healing, in Spencer S, Galloway DF (eds): Principles of Surgery, International edition. New York, McGraw-Hill Book Company, 1999, pp 263–290.
24. Svensjo T, Pomahac B, Yao F, Slama J, et al: Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg 106:602–612, 2000.
25. Erikson E, Perez N, Slama J, Page CP, et al: Treatment of chronic, nonhealing abdominal wound in a liquid environment. Ann Plast Surg 36:80–83, 1996.
26. Breuing K, Erikson E, Liu P, Miller DR. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res 52:50–57, 1992.
27. Dyson M, Young S, Pendle L, et al: Comparison of the effects of moist and dry conditions on dermal repair. J Invest Dermatol 91:434–439, 1988.
28. Vogt PM, Andree C, Breuing K, et al: Dry, moist, and wet skin wound repair. Ann Plast Surg 34:493–500, 1995.
29. Winter GD: Epidermal regeneration studied in the domestic pig, in Maibach HI, Rovee DT (eds): Epidermal Wound Healing. Chicago, Year Book Medical Publishers, Inc, 1972, pp 71–112.
30. Jonkman MF, Hoeksma EA, Nieuwenhuis P: Accelerated epithelialization under a highly vapor-permeable wound dressing is associated with increased precipitation of fibrin(ogen) and fibronectin. J Invest Dermatol 94:477–484, 1990.
31. Jonkman MF: Epidermal wound healing between moist and dry. Thesis, University of Groningen, Groningen, 1989.
32. Winter GD: A note on wound healing under dressings with special reference to perforated-film dressings. J Invest Dermatol 45:299–302, 1965.
33. Sawada Y, Yotsuyanagi T, Sone K: A silicone gel sheet dressing containing an antimicrobial agent for split thickness donor site wounds. Br J Plast Surg 43:88–93, 1990.
34. Winter GD, Scales JT: Effect of air drying and dressings on the surface of a wound. Nature 197:91–92, 1963.
35. Xu R: The medicine of burns and ulcers, a general introduction. Chinese J Burns Wounds Surf Ulcers (1):68, 1989.
36. Wang GS, Zhang YM, Liu RS, et al: Experimental study of the effect of MEBO on blood rheology in the treatment of burned rabbits. Chinese J Burns Wounds Surf Ulcers (4):30–32, 1993.
37. Li L: Experiment on inhibiting constriction of the ileum from a white mouse. Chinese J Burns Wounds Surf Ulcers (1):50–51, 1990.
38. Qu YY, Wang YP, Qiu SC, et al: Experimental research on the mechanism of the effect of MEBO. Chinese J Burns Wounds Surf Ulcers (4):4–9, 1997.
39. Qu YY, Wang YP, Qiu SC, et al: Experimental research on the anti-infective mechanism of MEBO. Chinese J Burns Wounds Surf Ulcers (1):19–23, 1996.
40. Xing D: Experimental study on the actions of the moist burn ointment on promoting healing of skin wound and anti-infection. Chinese J Burns Wounds Surf Ulcers (1):75–76, 1989.
41. Geng XL, Bu XC, Gao FQ, Liu YL: Study on the bacterial count in the subeschar living tissues of burn wounds. Chinese J Burns Wounds Surf Ulcers (1):49–50, 1989.
42. Huang QS, Zhou G, Su BP, Huang EX: A comparative study of fibronectin and MEBO in the treatment of experimental rabbit corneal alkaline burn. Chinese J Burns Wounds Surf Ulcers (1):18–19, 1995.
43. Wang GS, Jian WG, Xu XS, et al: The exploration of pathological changes and their mechanism of experimentally burned rabbits after treatment. Chinese J Burns Wounds Surf Ulcers (3):7–11, 1992.
44. Ang ES, Lee ST, Gan CS, et al: The role of alternative therapy in the management of partial thickness burns of the face—-experience with the use of moist exposed burn ointment (MEBO) compared with silver sulphadiazene. Ann Acad Med Singapore 29:7–10, 2000.
45. Chang CC, Kuo YF, Chiu HC, Lee JL: Hydration not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res 59:705, 1995.
46. Davy RB, Wallis KA, Bowering K: Adhesive contact media: an update on graft fixation and burn scar management. Burns 17:313, 1991.
47. Johnson JB, Ichinose H, Obagi ZE, Laub DR: Obagi’s modified trichloroacetic acid (TCA)-controlled variable-depth peel: A study of clinical signs correlated with histologic findings. Ann Plast Surg 36:225–237, 1996.
48. Obagi ZE, Obagi S, Alaiti S, Stevens MB: TCA-based blue peel: a standardized procedure with depth control. Dermatol Surg 25:773–780, 1999.
49. Ioannovich
J, Tsati E, Tsoutsos D, et al: Moist exposed burn therapy: Evaluation
of the epithelial repair process (an experimental model). Ann Burns Fire Disast 8:3–9, 2000.
Figure 1. (A)
Patient 1: healing of study and control areas at 8 weeks. Early epidermal
sliding is evident in the Sofra Tulle treated area.
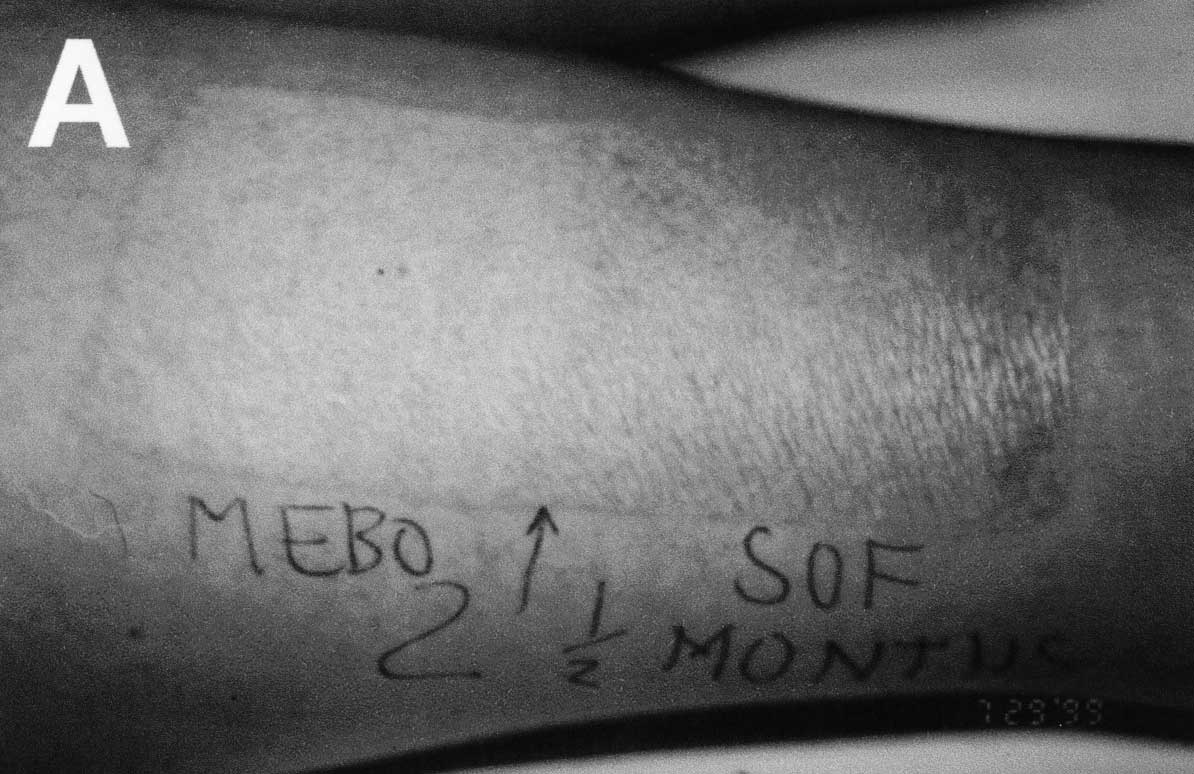
Figure 1. (B)
Patient 2: STSG donor site at 40 days. Note rapid regrowth of hair
in the MEBO treated area.
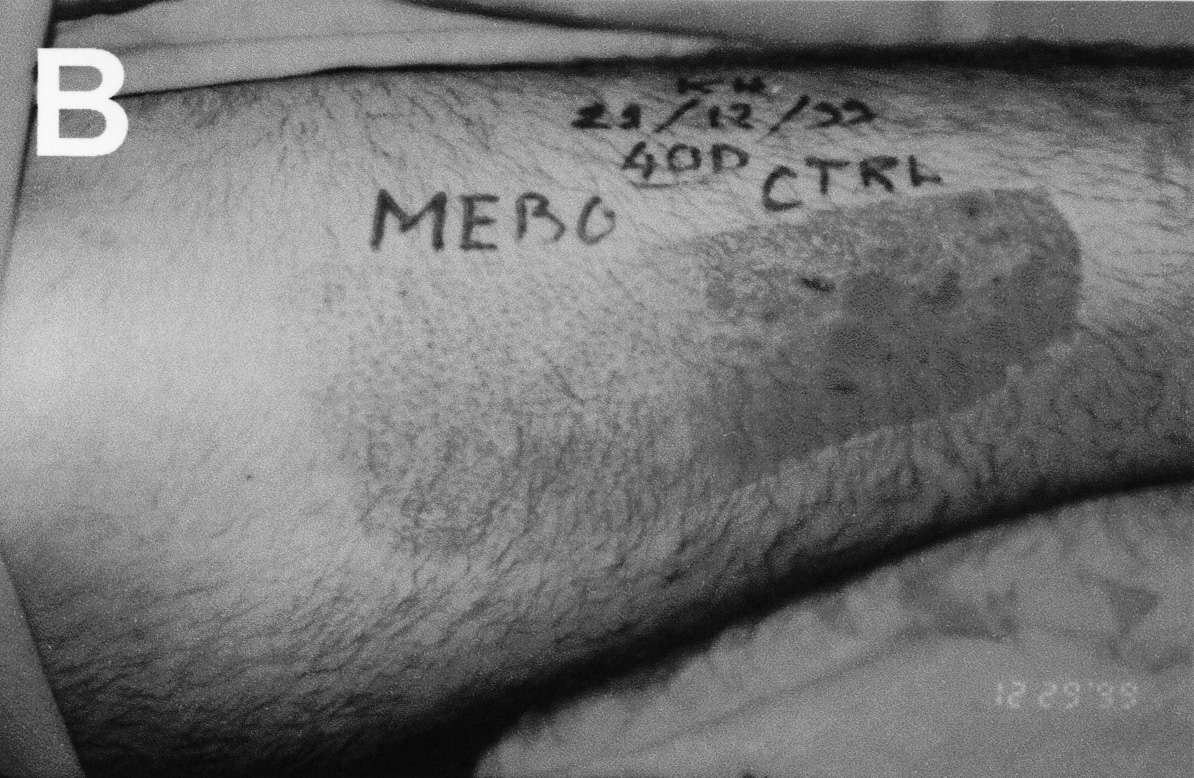
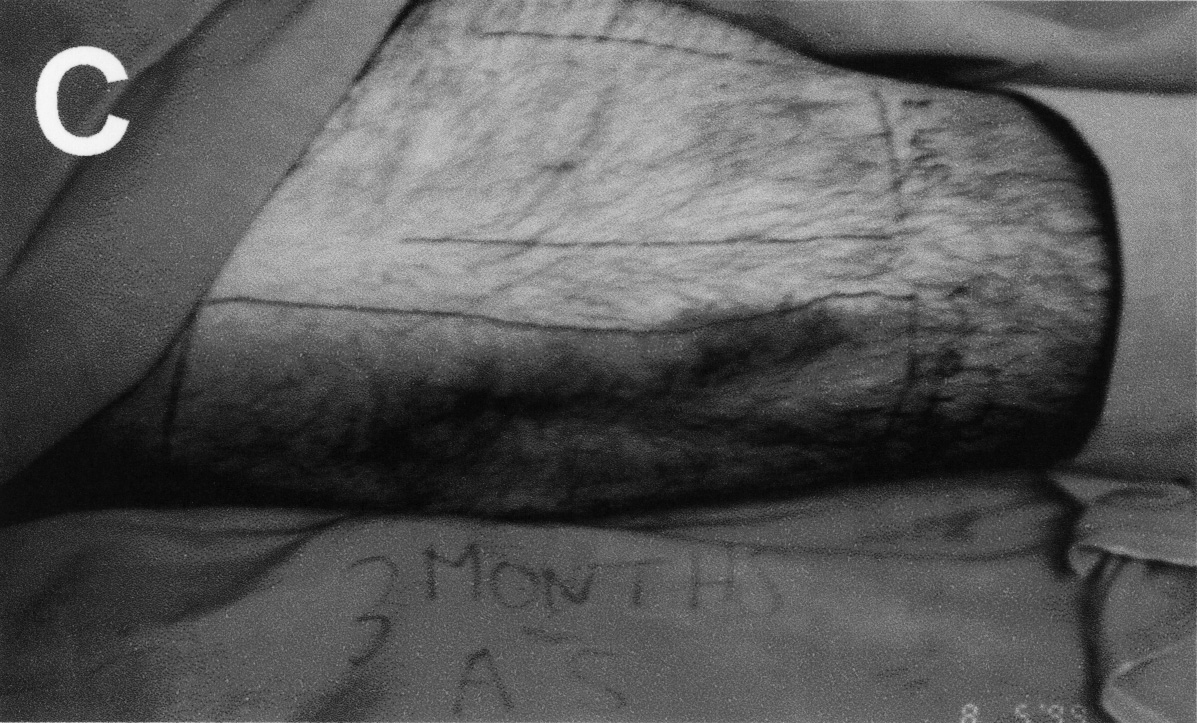
Figure 1. (C)
Patient 3: STSG donor site at 3 months. Severe hyperpigmentation with
moderate scar hypertrophy evident in the Sofra Tulle treated area.
©2000-2013. All Rights Reserved. Veterinary Solutions LLC
2Checkout.com is an authorized retailer for The
Journal of Applied Research